L-Aspartic Acid: Unpacking the Journey, Science, and Usages
Tracing L-Aspartic Acid from Discovery to Now
Long before biotech firms started optimizing amino acids, researchers working with plant proteins in the early 1800s stumbled across asparagine while isolating compounds from asparagus juice. Later, asparagine’s close relative, L-Aspartic Acid, emerged through systematic hydrolysis. The story of this amino acid stretches from that era of painstaking chemistry to today’s industrial fermenters. Back then, extracting L-Aspartic Acid was labor-intensive and required sleuthwork with what now seem like primitive tools. Now, high-yield processes rely on the precision of microbial fermentation, letting chemists produce it by the ton without endless rounds of extraction. I’ve spent time volunteering in a university lab where the sheer shift in efficiency upgrades and safety measures over these many decades stands out in every corner. L-Aspartic Acid has been part of the core set of amino acids found in living things since those early days, but its uses and the depth of research behind its effects have expanded far beyond anyone’s expectations in those dimly-lit chemistry rooms.
What Makes Up L-Aspartic Acid?
It’s a non-essential amino acid, which basically translates to our bodies being able to make it out of things we eat. That doesn’t take away from its importance—L-Aspartic Acid ends up shaping proteins, helps with energy cycles, and works its way into many nutritional supplements. Chemically, it carries the formula C4H7NO4. It crystallizes well, becoming a white and nearly odorless powder at room temperature. L-Aspartic Acid stays stable under normal storage conditions and dissolves well in water but not in most organic solvents, which makes sense given its biological context. It carries two carboxyl groups and an amino group, which gives it a slightly sour taste in pure form and makes it an active player in biochemical pathways that keep us alive.
Labeling, Specs, and Technical Guarantees
Companies who bring L-Aspartic Acid to the market don’t just fill up jars and slap labels; they give details about purity, moisture, and heavy metal content. In food, pharma, and research sectors, the story always centers on standards. The assays usually specify no less than 98.5% purity, low chloride and sulfate, lead at less than 1ppm, and microbiological purity guaranteed by negative pathogen tests. In my experience assisting in quality control, finding even micro-amounts of contaminants could set back weeks of work or invalidate a whole shipment. Each label not only spells out these minimum technical requirements but also offers storage tips to keep the product as close as possible to the numbers stated on arrival day.
Roads to L-Aspartic Acid: From Fermentation to Chemical Synthesis
Step into a commercial facility, and you’ll spot two main ways of making L-Aspartic Acid: fermentation or chemical synthesis. Fermentation, now the common choice, uses specific strains of bacteria to convert carbohydrates into the amino acid. Companies favor this route because it gives higher yields with fewer unwanted leftovers. Old-school chemists can still crank out L-Aspartic Acid from asparagine using acidic hydrolysis, but the complexity and environmental impact tend to make fermentation the smarter play. Behind the scenes, a modern fermenter keeps temperature, pH, and oxygen tight, so bacteria thrive without causing trouble later in the process. One big leap I’ve seen in recent years is the focus on reducing byproducts, so cleaner product ends up in every drum.
Chemical Reactions and Smart Modifications
Industries don’t just want L-Aspartic Acid as-is; they often need tweaks. Scientists coax it into becoming derivatives by swapping out hydrogen on the amino group or working with the carboxyl side chains. Aspartame, the familiar food additive, forms by hooking up L-Aspartic Acid and phenylalanine. Chemists can also set up amidation or esterification if unusual applications demand it. In my grad school experience, these modifications open a world of specialty chemicals, medical diagnostics, and advanced polymer resins that make L-Aspartic Acid central far outside biology. The pace of technical change has only picked up, as green chemistry principles steer new reactions to cut waste and energy use.
Nicknames and Market Names
The science world usually calls it L-Aspartic Acid or simply “Asp.” In ingredient decks and research logs, it also pops up as (S)-2-Aminobutanedioic acid, L-Amino-succinic acid, or even Aminosuccinic acid. No matter which name crops up, it stands for the same key amino acid, with applications from nutritional supplements to food industry formulas. Different regions may have their regulatory or supplier-specific synonyms, but what matters is the central molecule built around those functional properties.
Safety in Sourcing, Handling, and Working
L-Aspartic Acid generally handles safely in most lab and production settings. Standard operating procedures call for gloves, dust control, and eye protection, same as with lots of crystalline powders. In bottled form or as a food fortifier, it has a strong record of safe use. Guidelines from agencies like the FDA and EFSA set clear limits and outline smart precautions for operators and users. In my own shifts managing chemical supplies, rare problems came mainly from dust in the air or spills, handled by simple cleaning without drama. Clear labeling of containers and secure storage keep risks down to background levels. The push now is for manufacturers to extend these best practices all the way back to plant or fermenter operations, not just the final product.
Where Labs and Factories Use L-Aspartic Acid
Its best-known position comes in making aspartame, the global low-calorie sweetener found in dozens of foods. But L-Aspartic Acid’s story doesn’t end at the dinner table. Food companies bring it to the table as a nutritional booster. Sports supplement makers tout its roles in muscle metabolism and recovery formulas. Pharmaceutical players utilize it in intravenous nutrient solutions and even in certain non-toxic treatments. Specialty chemical firms blend it into biodegradable polymers and surfactants. Biomedical researchers have found critical roles in neurotransmitter pathways, which connect to deeper studies on brain function and fatigue. Take a walk through any science building and odds are you’ll find L-Aspartic Acid tucked away—sometimes as a basic ingredient, other times as a research probe or standard. Outside of industrial and lab settings, animal feed often includes a dose for livestock health. The common thread is its utility across many sectors, shaped by supply chain transparency and regulatory oversight.
Ongoing Research and Development
University groups and private R&D labs keep pushing ahead with inquiry about L-Aspartic Acid, focusing both on practical uses and the way it works inside living organisms. Advances in genetic engineering are helping organisms crank out higher yields. Researchers are also leveraging new reactors that use less energy and generate fewer polluting byproducts. Some teams study how aspartic acid supplementation affects endurance, cognitive performance, or metabolic cycles, while others use it to build smart polymers or specialty agents for targeted drug delivery. These projects attract funding because the potential upsides touch so many corners of health, sustainability, and materials science.
Examining Toxicity and Health Concerns
Safe use always gets the spotlight in ingredient science. Decades of animal and human tests haven’t linked standard intake levels with dangerous toxicity. Regulatory reviews confirm that dietary exposure poses minimal risk. Even so, extremely high doses—far beyond those ever recommended or eaten—can unsettle metabolic balance, especially in sensitive populations. Well-designed toxicological studies keep tracking outcomes in those few edge cases and confirm health standards with fresh data, not just outdated studies. From my observations in regulatory science, health authorities have a robust framework for evaluating new findings, so product safety isn’t just assumed—it’s continuously earned and reviewed.
What’s Next: Future Directions and Promise
L-Aspartic Acid’s future rises on better biotech production, cleaner supply chains, and expanding research into new uses. Work continues to lower the costs and environmental toll of making it while raising output per batch. Synthetic biology teams are programming even more efficient bacteria to crank out the acid from renewable feedstocks, potentially turning food waste into value. Downstream, as pharmaceutical and food tech innovators explore new bioactive derivatives and sustainable polymers, L-Aspartic Acid could anchor new product lines not envisioned a decade ago. Research on its deeper metabolic and neurological impacts may unlock better therapies and help personalize nutrition. The real story isn’t just that it’s an amino acid on a shelf, but that it bridges plant science, medicine, manufacturing, and our broader quest to balance nutrition, sustainability, and health.
What are the health benefits of L-Aspartic Acid?
Understanding What L-Aspartic Acid Does
L-Aspartic acid rarely gets mentioned outside technical or nutritional circles, but it plays a direct, hands-on role inside the body. It belongs to the group called amino acids—building blocks working to shape proteins, help shift energy, and keep the mind sharp. This amino acid shows up in foods like poultry, eggs, dairy, and some vegetables. So why bring up L-Aspartic acid, and what’s it doing every day in your body or on your plate?
Energy and Metabolism Powerhouse
L-Aspartic acid helps kick off the Krebs cycle, converting food into energy that fuels muscles and organs. Think of a day when you feel wiped out after a workout or heavy mental activity; your body keeps reaching for amino acids to refuel and repair. In my own exercise routines, I notice improvements in recovery speed and muscle endurance after meals high in protein, such as lean chicken or tofu—both containing aspartic acid and other essential amino acids. Scientific literature backs up that the Krebs cycle needs aspartic acid to run smoothly (see NIH research on cell metabolism). Without it, the energy “assembly line” gets jammed, slowing both mental and physical performance.
Supporting Brain Chemistry
L-Aspartic acid also acts as a neurotransmitter, connecting nerve cells and influencing brain function. My periods of mental fog or stress often follow irregular diets or skipped meals, hinting at the connection between nutrition and clarity of thought. Some animal studies point out that this amino acid can sharpen focus, boost alertness, and lift mood. In humans, research is still piecing together how much aspartic acid matters in daily cognition, but it’s clear that a well-fed brain runs better and feels less sluggish.
Boosting Hormone Production and Immune Response
In men, L-Aspartic acid helps regulate hormone production, especially testosterone. Research from small clinical trials has shown that supplementing with this compound leads to moderate rises in testosterone levels in men with low baseline levels. This does not mean everyone will notice large hormone surges, but the connection reminds us how essential building blocks like amino acids can support normal body functions. L-Aspartic acid may also help with immune cell production, as protein synthesis is necessary for immune response. Balanced diets often lead to better overall immunity, as seen during cold and flu season when people with higher protein intake can recover faster.
Food Sources Work Better Than Supplements
Packing more L-Aspartic acid into your diet doesn’t mean running to the supplement aisle. Whole foods such as lean meats, fish, eggs, lentils, nuts, and certain beans contain plenty. I keep hard-boiled eggs in the fridge for a quick snack, and it often provides a mid-afternoon boost, showing how food-based amino acids support lasting energy. Most people get enough aspartic acid from a varied diet, and nutrition authorities, such as the USDA, recommend relying on broad dietary sources rather than pills unless advised by a healthcare provider. Supplements sometimes help those with specific deficiencies or medical needs, but most folks benefit from eating a range of protein-rich foods.
Looking at Safety and Balanced Consumption
Consuming L-Aspartic acid through food rarely causes trouble. Overdoing supplements, though, can sometimes lead to imbalances or digestive discomfort. Always check with a medical provider before adding amino acid powders or capsules, especially if you have kidney disease or other chronic conditions.
How should I take L-Aspartic Acid supplements?
Understanding What L-Aspartic Acid Does
L-Aspartic acid shows up in a lot of protein-rich foods, and it plays a key role in your metabolism. Some fitness fans and bodybuilders try out supplements that contain aspartic acid to help support muscle recovery after workouts or for stamina. Some people point to benefits for energy and hormone levels, but research often comes mixed or limited.
Choosing a Supplement: Read the Label and Know the Source
Supplements sometimes seem like the wild west—uncertainty about dosages, mystery about sources. Products vary a lot. Finding a reputable brand makes a difference; third-party testing from places like USP or NSF gives more reliability. Check the ingredient list for fillers or extra additives you weren’t expecting. If a supplement hides behind a “proprietary blend,” skip it and pick one that’s straightforward about what’s in the capsule or powder.
Recommended Dosages: Play It Safe
Most people eating a balanced diet already get enough aspartic acid from steak, eggs, poultry, dairy, and beans. Supplement dosages run around 2–3 grams per day for adults. Start on the lower end to check tolerance—some users have seen digestive discomfort. Never go overboard. More doesn’t translate to bigger gains and too much could throw off your body’s chemistry. If you already eat a protein-rich diet, extra supplementation rarely adds benefits.
Best Practices for Timing and Absorption
A lot of supplements work best on an empty stomach, but aspartic acid can upset a sensitive gut. Splitting up the dose with meals may help you avoid bloating or cramping. With powders, mix into a drink that isn’t too acidic. Capsules go down fine with a glass of water. If you’re stacking supplements—say, protein powders and amino acids—spread them out so your system has an easier time processing everything.
Safety and Risks: Use Common Sense
Aspartic acid processed from foods rarely causes trouble. Large supplemental doses create risks, especially for folks with kidney disease or chronic health concerns. Check with your healthcare provider if you take medications or have underlying issues. No supplement replaces proper nutrition, sleep, and training. Try to avoid jumping after every social media-backed “miracle” or gym bro tip.
What the Research Says Now
Studies on L-aspartic acid raise more questions than answers. Improvements in testosterone or athletic performance often get debated. Large high-quality trials are rare. In my own experience and through conversations with lifters and athletes, few report major results directly linked to aspartic acid alone. If you’re tracking recovery or hormone levels, regular check-ins with a trusted doctor matter more than self-experimentation.
Smart Use: Listen to Your Body
Plenty of people achieve their fitness goals on food alone, and that’s what doctors recommend first. If you decide to experiment, track how you feel and look for side effects. Drop the supplement if you sense any issues. Share your plans with a registered dietitian or sports nutritionist if you want more personalized advice. Good health comes from basics: balanced diet, steady workouts, and common sense with anything extra you add.
Are there any side effects of using L-Aspartic Acid?
Understanding L-Aspartic Acid
L-Aspartic acid is one of the non-essential amino acids the body uses to make proteins. Some athletes and health enthusiasts turn to it, thinking it might help with energy or muscle growth, or maybe brain function. It shows up in every protein-rich food you can think of, like meat, eggs, beans, and nuts. Supplements focus on this single amino acid, promising specific benefits, but sometimes we forget there's always another side to the story.
Potential Side Effects from Experience
Adding a new supplement often brings hope. I’ve seen plenty of folks try amino acids for a fitness boost. Most just hope for better performance or faster recovery. Some don’t notice much effect, but every so often, small changes start to pop up. Gastrointestinal discomfort happens, like stomach cramps, bloating, or loose stools. It's easy to ignore these symptoms and blame something else, but they track back more often than we think to these new additions.
Taking more than the body needs can put stress on the digestive system. Once, during a busy training phase, I experimented with amino acid blends, including aspartic acid, and ended up feeling more run down than usual—nothing dramatic, just low energy and a little irritability. Turns out, too much can throw off balances of other amino acids, affecting mood or sleep. The body handles what it needs, but too much gets tossed aside or, worse, clogs up the works.
What the Research Says
Medical journals highlight that L-aspartic acid remains generally safe in normal diets. Scientific reports rarely show severe problems, especially in people with healthy kidneys and no metabolic issues. People with kidney disease or urea cycle disorders should stay away from extra amino acids—these conditions can make excess aspartic acid harmful since the body has trouble clearing it.
Studies haven’t turned up strong evidence that aspartic acid delivers major improvements in athletic performance. Some papers looked for hormone or strength boosts and came up mostly empty. Self-medicating because something is “natural” can bring unexpected results. Instead of health bumps, I’ve seen regular folks feel jittery, sleep poorly, or feel more anxious while taking high doses.
Why Paying Attention Matters
Supplements work best when used with a plan. Chasing gains or energy with single amino acids skips over the complexity built into whole foods. Relying on protein-rich meals rather than single supplements creates a steady, reliable intake. Every time a health trend shows up, my tendency is to look back at the basics—balance, moderation, and consistency trump most quick fixes.
Many side effects are mild, but some people face allergies or unknown interactions with meds. Reading labels and talking to a healthcare provider always pays off. Problems rarely start with a healthy diet—issues almost always show up once we stack extra, unnecessary products on top of it.
Looking Forward
Responsible supplement use starts with a clear question: “Do I really need this?” In most cases, the answer points back to real food. Chasing after minor boosts with isolated ingredients like L-aspartic acid often adds more worry than reward. Simple dietary changes can outdo any pill or powder, with fewer surprises down the line. Listening to the body and seeking science-backed advice protects health far more than quick trends.
Is L-Aspartic Acid safe for long-term use?
Understanding L-Aspartic Acid
L-Aspartic Acid shows up in most protein-rich foods. It’s an amino acid most people eat daily without realizing, found in dairy, beef, chicken, eggs, fish, and even plant sources like oats and asparagus. The human body actually produces it, using it to build other amino acids and make chemical messengers for the brain. For years, fitness fans have picked it for supplements, especially those chasing muscle gains, but even folks not looking for bulging biceps pick it for possible cognitive benefits.
What Long-Term Use Really Means
Stories about anything you put in your body for months—or years—raise questions. Regular, high-dose use isn’t the same as getting it in a steak or an omelet now and then. In most people, eating aspartic acid from a regular mixed diet hasn’t rung any alarm bells. The body breaks down any extra and flushes it out, so healthy kidneys usually don’t let levels build up.
Supplements tend to crank up the dosage, and here is where people should slow down. No major dangers flash in the research for moderate use, but ramping up many-fold with pills brings the unknown. Researchers have looked at doses around 3 grams per day for a few weeks and noted little more than mild stomach trouble for some. They haven’t thoroughly mapped out what happens with high levels over many years, and that uncertainty leaves plenty of room for caution.
What the Research Tells Us—And What’s Missing
Most data comes from animal studies or short, small human trials. Animal results do suggest aspartic acid plays a role in hormone production, such as helping signal testosterone or affecting nervous system health. In people, there’s yet no strong proof of harm, but also no convincing evidence for dazzling benefits advertised by supplement companies.
People with kidney disease, liver trouble, or who take certain medications face more risks. For these groups, taking in lots more of any amino acid skews the balance the body works so hard to keep. Doctors warn against using unproven supplements if there’s already a medical condition muddying the water.
Sensible Use Based on What We Know
Long-term safety, in this case, often boils down to how much a person uses, what else they might be taking, and if they already have medical issues that could make their body less efficient at processing amino acids. Regular check-ins with a healthcare provider help catch any unexpected changes in kidney or liver function, which are key players in handling amino acids like aspartic acid.
It pays to treat L-Aspartic Acid supplements with the same respect as any concentrated nutrient—more doesn’t always mean better. Taking careful note of amounts and staying within ranges found in food makes sense until long-term research clears up the picture.
Better Information, Better Decisions
Supplements offer promises, but the science often lags behind. Real answers about 10 years of higher-dose aspartic acid in healthy adults just don’t exist yet. Until large, independent studies track people for longer stretches, the safe bet is eating balanced meals, letting the body use nutrition the way it’s built for, and speaking with a trusted medical professional before picking up any high-dose supplement.
Can L-Aspartic Acid help with muscle growth or athletic performance?
Looking at the Hype Around L-Aspartic Acid
Folks on gym forums and supplement bottles love to talk up L-aspartic acid. Promoters say it helps muscles recover, boosts strength, and even lifts performance. Digging into this, it’s easy to see why the supplement world jumped on the bandwagon. Our muscles need amino acids to recover after hard workouts, and some amino acids play bigger roles than others. For athletes chasing an extra edge, any new promise feels worth a shot.
What Does Science Actually Say?
Our bodies already make plenty of L-aspartic acid during normal metabolism. You can also get it by eating foods like asparagus, sprouting seeds, or lean meat. The interest comes because L-aspartic acid helps shuttle other amino acids around, plays a part in the energy cycle, and supports the urea cycle, which helps get rid of ammonia during tough workouts.
Scientific studies so far haven’t shown a big muscle-building effect from taking extra L-aspartic acid. Reviews from journals like Nutrition & Metabolism and the International Journal of Sport Nutrition didn’t find evidence that it adds to muscle growth. These reputable sources note amino acids like leucine and glutamine have a direct hand in building and repairing muscle after heavy exercise, but aspartic acid doesn’t stimulate muscle protein synthesis the same way.
Stories from the Weight Room
A few years ago, I tried a blend of amino acid powders, including L-aspartic acid, after reading a glowing social media post. I expected quicker recovery and stronger lifts. What I actually noticed? Nothing that stood out compared to whey protein, extra sleep, or regular food. And I’m not alone on this. Other lifters, even those tracking numbers closely, report more success sticking to basic nutrition: protein, enough carbs for energy, and sometimes creatine. Supplements with L-aspartic acid ended up sitting in the cupboard.
Potential Downsides and Better Solutions
Supplements cost money, and overdoing single amino acids can create imbalances in how your body absorbs other ones. Too much aspartic acid sometimes causes gut trouble, headaches, or mood shifts. For people who already eat a balanced diet, their muscles get all the L-aspartic acid they can use from everyday food.
If someone wants real muscle growth or better performance, the basics still stand tallest. Eat enough complete protein, get good carbs and fats, and lift steadily with a plan. Sleep repairs muscle as much as post-workout nutrition does. For edge-seekers looking to science, strong evidence shows that protein timing, branched-chain amino acids (especially leucine), and creatine actually move the needle for most people.
Anyone tempted by supplement promises would do best to check credentials—not just on the label, but in the pages of peer-reviewed journals. Talking to a registered dietitian or sports nutritionist can save cash and frustration. Athletic gains are stubborn: shortcuts rarely pay off as advertised, and basic habits win every time.
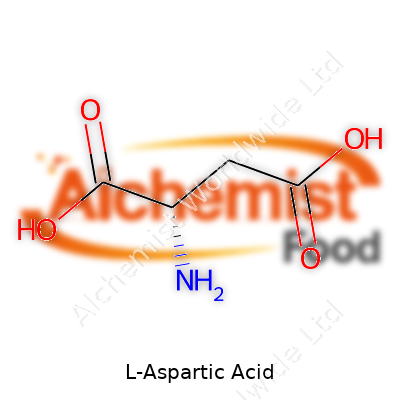
| Names | |
| Preferred IUPAC name | (2S)-2-aminobutanedioic acid |
| Other names |
Aspartic acid Aspartate |
| Pronunciation | /ˌel əˈspɑːrtɪk ˈæsɪd/ |
| Preferred IUPAC name | (2S)-2-aminobutanedioic acid |
| Other names |
Aspartic Acid L-Aminosuccinic acid L-Aspartate L-Aspartate acid L(-)-Aspartic acid |
| Pronunciation | /əˈspɑːrtɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 56-84-8 |
| Beilstein Reference | 87853 |
| ChEBI | CHEBI:29961 |
| ChEMBL | CHEMBL601 |
| ChemSpider | 565 |
| DrugBank | DB00191 |
| ECHA InfoCard | ECHA InfoCard: 100.003.478 |
| EC Number | EC 200-733-8 |
| Gmelin Reference | 65365 |
| KEGG | C00049 |
| MeSH | D001220 |
| PubChem CID | 596 |
| RTECS number | AS5950000 |
| UNII | W6DH1PM79L |
| UN number | UN1872 |
| CAS Number | 56-84-8 |
| Beilstein Reference | 69272 |
| ChEBI | CHEBI:29961 |
| ChEMBL | CHEMBL975 |
| ChemSpider | 585 |
| DrugBank | DB00132 |
| ECHA InfoCard | 100.032.039 |
| EC Number | EC 200-564-8 |
| Gmelin Reference | 6048 |
| KEGG | C00049 |
| MeSH | D001217 |
| PubChem CID | 596 |
| RTECS number | CI2325000 |
| UNII | X0CG6XQVQK |
| UN number | UN9077 |
| Properties | |
| Chemical formula | C4H7NO4 |
| Molar mass | 133.10 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.66 g/cm3 |
| Solubility in water | sparingly soluble |
| log P | -3.89 |
| Acidity (pKa) | 2.09 |
| Basicity (pKb) | 3.89 |
| Magnetic susceptibility (χ) | -26.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.630 |
| Dipole moment | 6.0057 D |
| Chemical formula | C4H7NO4 |
| Molar mass | 133.10 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.66 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -3.89 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.90 |
| Basicity (pKb) | 2.77 |
| Magnetic susceptibility (χ) | -27.2×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.63 |
| Dipole moment | 9.3746 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 166.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1081.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1579 kJ/mol |
| Std molar entropy (S⦵298) | 111.0 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1002.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1415 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | A13AA03 |
| ATC code | A16AA08 |
| Hazards | |
| Main hazards | Irritating to eyes, respiratory system, and skin. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| NFPA 704 (fire diamond) | 1-1-0-W |
| Flash point | 233°C |
| Autoignition temperature | 410 °C |
| LD50 (median dose) | LD50 (median dose): Mouse, oral: 10930 mg/kg |
| NIOSH | SY8270000 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 10-30 g |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 324.8°C |
| Autoignition temperature | 430 °C |
| Lethal dose or concentration | LD50 oral rat 4520 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral rat: 4,510 mg/kg |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 2.66 g |
| Related compounds | |
| Related compounds |
Aspartate N-Acetyl-L-aspartic acid L-Glutamic acid L-Asparagine β-Alanine |
| Related compounds |
Glutamic acid Asparagine Succinic acid Fumaric acid Malic acid Oxaloacetic acid L-Alanine |

