Iron Pyrophosphate: A Closer Look from Origins to Future Applications
Historical Development
Iron pyrophosphate has roots in the early days of inorganic chemistry, showing up in research and industry for generations. Chemical pioneers explored its utility long ago, both for its distinctive properties and for the growing need to supply iron in stable forms to food, pharmaceuticals, and industry. In the twentieth century, industry geared up to produce iron pyrophosphate on larger scales. Food fortification, especially bread, cereal, and infant formula, gave this compound a steady presence on ingredient lists in many countries. Curious minds in science looked well beyond nutrition, with researchers probing its stability in water and its compatibility with other additives. The legacy connects the scientific curiosity of the past with everyday consumer needs.
Product Overview
Iron pyrophosphate brings a practical solution where soluble and reactive iron salts create problems. Its use extends to dietary supplements, food fortification, some fertilizers, and laboratory reagents. Manufacturers often ship it as a white or grayish powder. In nutritional products, the aim focuses on delivering iron in a gentle, non-reactive way — especially when direct taste, oxidation, or color changes from other iron salts become a hurdle. Companies in the US, Europe, and Asia produce iron pyrophosphate under regulatory frameworks for both food and pharma products, placing product quality and consistency at the top of their lists.
Physical & Chemical Properties
Iron pyrophosphate, with the chemical formula Fe4(P2O7)3, forms as a fine powder, not quite soluble in water. It resists changes in pH and holds up in conditions where other iron sources turn unstable or unpleasant. Standard grades do not carry strong odor or taste, making it easier to use in foods where flavor matters. Compared to ferrous sulfate or gluconate, iron pyrophosphate does not speed up fat rancidity or discoloration of light bakery products. Its color stays light under routine storage. With a melting point well above 400°C, there is little risk of decomposition at typical usage temperatures.
Technical Specifications & Labeling
Producers of iron pyrophosphate supply detailed certificates covering iron content, loss on drying, soluble phosphate, lead, arsenic, and other heavy metal levels. Food regulatory authorities such as the FDA, EFSA, and China’s National Food Safety Standard track purity standards to guarantee safety. Package labels name the product as “Ferric Pyrophosphate” or simply “Iron(III) pyrophosphate,” mentioning CAS Number 10058-44-3. Plain packaging identifies the batch, expiry, iron percentage, and food– or pharma–grade status. In nutritional products, legislation often dictates maximum allowable levels to avoid iron overload. Brand names range from generic chemicals to trade-marked blends with targeted dispersibility or granulation.
Preparation Method
Industrial production starts with iron salts such as ferric chloride or ferric sulfate, which react with soluble phosphate sources, usually sodium pyrophosphate, under controlled pH and temperature. Equipment keeps the reaction mixture well-stirred, preventing clumping. The resulting iron pyrophosphate solidifies as a suspension. Filtration and drying finish the process, and the powder undergoes sifting to deliver desired mesh size. For better suspension in beverages or infant formula, factories use spray dryers or specialized granulation methods, yielding improved dispersibility.
Chemical Reactions & Modifications
Iron pyrophosphate shows little solubility in water, sticking to its form unless exposed to acidic or reducing environments. In acidic pH, for instance in the stomach, it releases iron ions that the human body can absorb. Researchers explored its use as a catalyst or precursor in ceramics and metal treatment. Surface modifications, such as coating with lecithin or polysaccharides, improve the powder's ability to mix with liquids, an advance for the dairy and non-dairy beverage industries. Reactions with strong acids or reducing agents break down the structure, liberating iron; this is the core mechanism for iron supplementation.
Synonyms & Product Names
Alongside standard chemical names like “Ferric pyrophosphate” and “Iron(III) pyrophosphate,” the industry recognizes short forms such as “EHDP Ferric salt” or “Ferric pyrophosphoric acid salt.” Product names blend technical terminology and marketing, ranging from straightforward descriptors in chemical catalogs to proprietary blend names in consumer nutrition. Pharmaceutical suppliers differentiate between technical- and food-grade forms, both referencing international standards.
Safety & Operational Standards
Handling guidelines follow the usual care for fine powders — dust control, proper ventilation, and basic chemical safety measures such as gloves and eye protection. Iron pyrophosphate earns a spot on the FDA’s GRAS (Generally Recognized As Safe) list at nutritional levels, but factory staff keep Material Safety Data Sheets (MSDS) close to address risks like inhalation or accidental ingestion of bulk quantities. Newer guidance from the EU emphasizes tight limits on contaminants, given the compound’s use in infant nutrition. Storage in dry, sealed containers prevents caking and preserves long-term stability.
Application Area
In the food industry, iron pyrophosphate makes a difference in fortified cereals, bread, dairy, plant–based drinks, and even meal replacement powders. The challenge in these products revolves around disguising iron’s notorious taste and preventing color shifts. In the animal nutrition field, it finds space in enriched feed products. Laboratory researchers use it as an iron source in media or as a test reagent. Potential also exists for use in controlled-release fertilizer blends, exploiting its low water solubility for slow nutrient delivery. Pharmaceutical tablets designed for gentle iron supplementation frequently list iron pyrophosphate for patients with sensitive stomachs or for pediatric use.
Research & Development
Recent research zeroes in on better absorption in the body, since iron pyrophosphate avoids many of the digestive issues linked to traditional iron salts but absorbs less efficiently. Some teams coat iron pyrophosphate with emulsifiers or package it in nano-sized clusters to help the gut pick up more of the mineral. Universities and private labs run clinical studies comparing it with other iron sources, tracking indicators like hemoglobin improvement, side effects, and interactions with common foods. Scientists examine its effects on shelf stability in breakfast cereals, yogurt, or meal shakes, always searching for ways to supply iron without sacrificing flavor or texture.
Toxicity Research
Extensive animal and human toxicology work gives iron pyrophosphate a robust safety profile in line with established supplement guidelines. Iron toxicity primarily threatens those who consume huge doses over prolonged periods, or people with inherited disorders like hemochromatosis. Published reviews point to a wide safety margin at normal usage levels in food and supplements. The compound stays mostly inert during storage, lowering worries about degradation products or off-flavors. Ongoing studies test infant exposure and chronic intake over a lifetime to track for subtle risks not caught by older studies.
Future Prospects
Demand for iron supplementation in food and beverages shows no signs of shrinking, especially as plant–forward diets gather steam and iron deficiency remains stubborn worldwide. Iron pyrophosphate, with tweaks in particle size, coatings, or delivery matrix, brings hope for higher absorption and lower cost in next-generation fortification. Companies test blends with vitamin C or natural chelators to boost bioavailability, knowing that iron needs careful handling to skirt both deficiency and overload. Academic labs and regulatory bodies continue to watch for long-term health markers as new versions roll out in infant and adult health products. With environmental pressures mounting, the low environmental footprint of iron pyrophosphate production and usage fits the push for safer, greener ingredients across the board.
What is Iron Pyrophosphate used for?
The Real Reason Iron Pyrophosphate Matters
Most people pass by ingredient labels on cereal boxes or nutrition bars without paying much attention. I have done this countless times, only to circle back when my doctor flagged a mild iron deficiency. Out of curiosity, I took a closer look at what makes food “fortified with iron” and why iron pyrophosphate pops up on so many labels.
What Sets Iron Pyrophosphate Apart?
Iron comes in many forms. Unlike the type that rusts your garden fence, iron pyrophosphate looks like a fine, white powder. It is a compound that has steadily gained traction in the world of food science, especially in processed or fortified products. Anyone who has ever bitten into breakfast flakes or sipped on meal shakes might have encountered iron pyrophosphate, often unknowingly.
This form of iron allows food producers to add essential minerals to foods that would otherwise lack them. It makes sense: many processed foods lose natural iron during manufacturing. Adding it back helps prevent common problems like iron deficiency anemia, which can cause tiredness, weakness, and problems focusing, especially in children and pregnant people.
Why Not Just Use Plain Iron?
Iron pyrophosphate doesn’t dramatically change the taste, smell, or color of food. That’s a huge advantage for food companies. Traditional iron salts, such as ferrous sulfate, can make foods taste metallic or look odd. Distracting flavors have put people like me off iron supplements before. By choosing iron pyrophosphate, manufacturers protect both flavor and nutrition, so there’s less risk of consumers noticing a difference.
Tackling Nutritional Gaps
Iron deficiency remains one of the most common nutrition problems worldwide, not just in developing countries but also in areas with plenty of food choices. According to the World Health Organization, about 30% of the global population has anemia, with iron deficiency as a leading cause. Getting enough iron from diet alone is tricky, especially for vegetarians and those avoiding red meat. Iron pyrophosphate steps in to fill that gap, bridging the divide between convenience and health.
One thing you learn raising kids or cooking for a family is that nutrition sneaks in the back door. Most kids won’t eat spinach every meal. Fortifying pasta, flours, and snacks with iron pyrophosphate helps boost iron intake without the battles that come with supplements or unfamiliar foods.
Improving Iron Delivery for All
There’s always talk about how some forms of iron absorb better than others. It’s true that iron pyrophosphate isn’t the most easily absorbed, but research on microencapsulation and careful blending is helping to close that gap. Pairing iron-rich foods with vitamin C—like orange juice with cereal—makes absorption easier. More work in this area means fortified foods can help more people without leaving a chemical aftertaste.
I’ve seen firsthand how iron fortification brings peace of mind to families worried about nutrition. Advances in food technology, good research, and smart labeling all work together to make iron pyrophosphate a staple in better health. If you’re watching your iron or supporting someone who is, understanding these ingredients can make those food choices a little less mysterious.
Is Iron Pyrophosphate safe for consumption?
What Goes Into Our Food Matters
The food industry keeps searching for new ways to tackle iron deficiency, and iron pyrophosphate stands among the growing list of options. Anywhere iron-fortified cereals show up, there’s a good chance this ingredient helped put iron into the mix. People want reassurance that what lands on their breakfast table has been through proper checks and balances.
How Iron Pyrophosphate Finds Its Way Into Diets
Iron deficiency turns up as a big health issue worldwide. Solutions include food fortification, giving everyone a better shot at hitting their daily iron targets. Iron pyrophosphate holds an advantage: it doesn’t turn flour or cereal grey like some iron salts do, has little taste, and doesn’t mess with the food’s flavor. Its low solubility does slow down absorption, but for folks who’d otherwise be left out, it still moves the needle.
Safety Backed by Science
Looking at trusted scientific reviews, iron pyrophosphate consistently ends up marked as safe. The U.S. Food and Drug Administration lists it as GRAS—Generally Recognized as Safe. It has gone through toxicity studies that found no harmful effects, even over long-term intake far above what an average person would encounter through fortified foods.
A research review by the European Food Safety Authority looked at how the body handles iron from many sources and didn’t flag major worries for healthy people. Ascorbic acid (vitamin C) can help support better absorption, and this works as true for iron pyrophosphate as for other kinds of iron added to foods.
Are There Any Drawbacks?
Some might worry about not getting enough iron from any form that doesn’t dissolve quickly in the gut, and iron pyrophosphate mostly fits that bill. It’s true—less of it gets absorbed compared to forms like ferrous sulfate. In places with high rates of anemia, that lower absorption matters. Makers sometimes compensate by adding more, so the final product gives nearly the same iron boost.
There’s always a risk that folks with rare conditions like hemochromatosis, who store too much iron, might run into health problems if they take in too much. For most people, though, the body does a solid job controlling how much iron gets absorbed.
Solutions and Smart Choices
People have reasons to want straightforward answers about what they eat. Clear labeling on packaged foods would help build this trust. Manufacturers and regulators both ought to continue sharing new evidence as it comes in, making it easier for consumers to navigate their choices. Keeping schools and clinics in the loop helps everyone—from parents planning meals to doctors advising at-risk patients.
Iron pyrophosphate isn’t a silver bullet, but it plays a useful part in the bigger push to cut iron deficiency rates. Through decades of safe use around the globe and ongoing research, there’s strong evidence pointing to its safety in regular diets. A balanced menu remains the best bet for long-term health, with fortified foods lending needed support when gaps appear.
What are the side effects of Iron Pyrophosphate?
What Iron Pyrophosphate Really Does
Iron pyrophosphate, often found in breakfast cereals and supplements, supplies a form of iron that’s supposed to raise iron levels in people who feel run-down or have anemia. Iron matters for everyone, since not getting enough means feeling tired, weak, or unable to focus. Plenty of folks take iron supplements without thinking twice, but every ingredient brings its own quirks. My own brush with iron supplements showed me that not every type feels the same in your gut or in your day-to-day routine.
Common Gut Reactions to Iron Pyrophosphate
Many complain almost right away about belly upset. Nausea, gassy feelings, and cramps aren’t uncommon. If you deal with sensitive digestion—or already have irritable bowel symptoms—iron pyrophosphate can tip your gut over the edge. Constipation stands out as the side effect most people notice. Some experience the opposite: diarrhea, which can sneak up fast or last longer than you expect. Keeping hydrated and eating fiber can help, but sometimes a switch in supplement makes all the difference.
Changes in Stool and What They Mean
Anyone who’s taken oral iron before probably expects the change in stool color. Iron pyrophosphate often turns stool dark, almost black sometimes. This surprises a lot of people, but it’s not usually dangerous. The real worry comes with symptoms such as stomach pain paired with blood in the stool, which needs a doctor’s attention—and fast. If things don’t look or feel right, listen to your gut and talk to a professional.
Rare but Serious Reactions
Some folks develop allergic reactions—itchy rashes, swelling, breathing problems. Such events don’t happen much, but skipping a call for emergency help isn’t smart if swelling or shortness of breath shows up. Beyond that, too much iron carries longer-term risks. The body stores excess iron in ways that can hurt the liver, heart, and other organs. People with certain conditions—such as hemochromatosis—develop iron overload much more easily. Blood tests tell the real story. If you don’t know your iron status, taking more might harm more than help.
Why Personal Health History Shapes Everything
Some can swallow iron pyrophosphate with barely a burp, while others wind up with days of stomach issues or stained teeth. Women, young children, and those with chronic conditions notice side effects even more. The way the body absorbs this kind of iron can change if you throw in calcium-rich foods, coffee, tea, or even antacids—all regular parts of daily eating. If someone’s already using medicine or has complicated health, combining iron with other treatments gets tricky.
How to Keep Problems Small
Respecting the label and not doubling doses helps. Iron doses used in studies often sit higher than what’s found in fortified food, so don’t fear everything you read online. Spreading smaller doses across the day appears to lower the risk of gut drama. Eating a variety of foods with natural iron allows most people to avoid supplements entirely. If doctor’s advice says take iron, ask about dose, timing, and interactions. Lab work gives answers that guesswork can’t match.
Trust but Verify—The Best Move for Iron Supplements
Every supplement promises a quick fix, yet bodies don’t run on promises. If you start iron pyrophosphate and feel worse, trust what your body says. Real stories—including my own—show that the right iron source and dose make a world of difference. Most people just need a little knowledge and a lot of honesty with themselves. Iron is vital, but it never pays to ignore what makes you feel off.
How is Iron Pyrophosphate different from other iron supplements?
Understanding the Differences Matters
Choosing an iron supplement seems simple on the surface, but digging a little deeper can save a lot of trouble down the road. Iron shortages easily slip under the radar—until they start sapping energy and messing with health. Doctors hand out prescriptions for standard ferrous sulfate or ferrous fumarate all the time, but iron pyrophosphate grabs more attention in recent years, especially in food fortification and for people with sensitive stomachs.
Gentler on the Gut
Anyone who’s ever had to take iron pills on a regular basis knows they can hit the stomach like a ton of bricks. Nausea, constipation, and stomach cramps are common complaints. These problems often drive people to stop taking their supplements, even if they know they need them. Iron pyrophosphate changes the story. Its unique chemical structure makes it less soluble in the stomach’s acidic environment, so it doesn’t release as much free iron into the digestive tract all at once. For many folks, that means far fewer digestive issues.
Absorption Isn’t Always the Enemy
The big knock against iron pyrophosphate centers on absorption. Nutrition textbooks tend to tout the superior absorption of ferrous sulfate or gluconate. Those forms do go straight into action in the body, and that works well for folks who tolerate them. Iron pyrophosphate gets a lower score in old-school lab tests, but that doesn’t tell the whole story. Every day, millions eat foods fortified with this gentler iron source—breakfast cereals, certain meal replacements, even infant formula—because it changes taste less and causes fewer issues. Recent research points out that with better formulations, such as micronized versions, the body takes up iron pyrophosphate more effectively than outdated studies would suggest. FDA has recognized its safety for fortification, and experience shows it does the job for populations who can’t use stronger forms.
Taste and Color Considerations
Taste matters. Anyone who’s ever choked down a metallic-tasting iron pill knows that. Iron salts, especially in liquid form, can make drinks or foods taste harsh or turn odd colors. Try convincing a child—or even an adult—to drink a glass of water that looks rusty. Iron pyrophosphate sidesteps this issue. It’s much less reactive, so it doesn’t change the flavor, smell, or appearance of food products. That explains why so many companies trust it in granola bars, drinks, and kids’ cereals.
Matching the Right Iron to the Individual
One truth stands out in real life—no one supplement fits every person. Some people absorb iron like a champ, while others struggle. Those with inflammatory bowel issues or sensitive digestion often have the toughest time with standard iron pills, ending up right back where they started. In these cases, iron pyrophosphate gives their bodies enough iron to build up stores over time, without turning every meal into a struggle. It brings a solution to tables and schools where compliance matters more than numbers in a textbook.
Looking at the Bigger Picture
The world still faces widespread iron deficiency, especially in young children and women of childbearing age. Iron pyrophosphate expands the toolbox for tackling this challenge. No supplement will work miracles on its own. Getting enough vitamin C helps the gut grab more iron, regardless of form. Good diets and education always matter. As food science moves forward and demands more options, iron pyrophosphate stands out as a reliable choice for adding iron in ways that work for daily life—without the unpleasant side effects that steer people away from getting the nutrients they need.
What is the recommended dosage of Iron Pyrophosphate?
Iron’s Role in the Body
People sometimes overlook how much the body runs on iron. Just like a car sputters without gas, our muscles and organs slow down without enough iron. Iron pyrophosphate steps in for folks needing a boost, especially those fighting anemia or keeping up during a demanding pregnancy. As someone who once had to tackle low iron counts, I know a supplement’s reputation comes second to how well it works and how it fits into the rhythm of your meals and routines. Iron pyrophosphate appears mostly in foods or supplements looking for gentler iron delivery, often chosen for its mild taste and low stomach irritation.
Recommended Dosage: Numbers That Matter
Doses change a lot depending on age, condition, and diet. Adults dealing with iron deficiency commonly turn to daily doses ranging anywhere from 10 mg up to 60 mg of elemental iron, depending on the doctor’s guidance and the product itself. Pregnant women need more; up to 30 mg a day tends to be just right, since their blood supply runs overtime to make a healthy baby. Kids’ needs swing lower, commonly between 7 to 15 mg a day.
Iron pyrophosphate brings a steady hand because it enters the body slowly. This quality can help folks dodge the classic stomach aches that tend to come with quicker-absorbing supplements. The World Health Organization points out that regular iron intake above 45 mg can sometimes lead to side effects unless handled by medical advice, so dosing with respect is crucial.
Why Sticking to the Right Dose Matters
Iron piles up in the liver if a person takes too much. I learned this firsthand watching a friend battle iron overload from ‘just in case’ supplements. Nausea, joint pain, and even long-term harm become very real risks. On the flip side, too little iron brings fatigue, brain fog, and restless legs at night. Getting that number from a health professional – not just a store shelf guessing game – matters more than most folks realize.
Diet also nudges the need for iron up or down. Meat eaters absorb iron better than plant-focused people. Vitamin C helps the body unlock more iron from every capsule, so a glass of orange juice with your supplement does more work than a pill on its own.
Trusted Sources Matter
Clear dosage advice always starts with honest conversations between patient and clinician. Groups like the Centers for Disease Control and World Health Organization update iron recommendations as new facts roll in, since nutrition science keeps shifting. A quality supplement won’t hide its ingredients or dosage—labels should match the advice you hear in the doctor's office.
A pharmacist once reminded me: “Iron is essential, but taking too much is never better than just enough.” This principle serves me well today. Sticking to evidence-based numbers, keeping tabs on your blood tests, and consulting your care team keeps guesswork at bay.
Improving Iron Supplement Safety
No one should feel confused choosing supplements. Clear labeling and better education around the kitchen table would go a long way. Health classes, primary care visits, and public health campaigns can all help spread accurate dosing information. Ultimately, everyone deserves safe, effective solutions backed by research, not fads or wishful thinking. For anyone starting iron pyrophosphate, a check-in with a real human expert always beats going it alone.
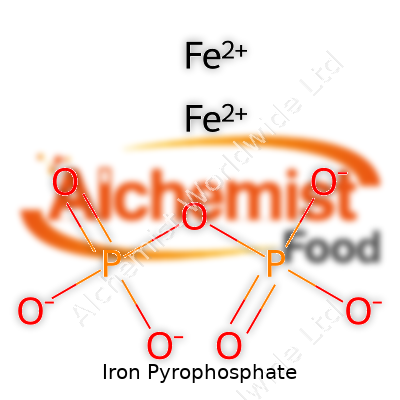
| Names | |
| Preferred IUPAC name | Iron(3+) diphosphate |
| Other names |
Ferric pyrophosphate Iron(III) pyrophosphate Ferripyrophosphate |
| Pronunciation | /ˌaɪərn paɪˈrɒfəˌsfeɪt/ |
| Preferred IUPAC name | Iron(3+) diphosphate |
| Other names |
Ferric pyrophosphate Iron(III) pyrophosphate Pyrophosphoric acid, iron(3+) salt Ferripyrophosphate |
| Pronunciation | /ˈaɪərn paɪ.rəˈfɒs.feɪt/ |
| Identifiers | |
| CAS Number | [10058-44-3] |
| Beilstein Reference | 3580631 |
| ChEBI | CHEBI:30413 |
| ChEMBL | CHEMBL1201640 |
| ChemSpider | 142030 |
| DrugBank | DB11271 |
| ECHA InfoCard | 03-2119561488-42-0000 |
| EC Number | 233-190-0 |
| Gmelin Reference | 78523 |
| KEGG | C18602 |
| MeSH | D017363 |
| PubChem CID | 24857 |
| RTECS number | TT8970000 |
| UNII | SG1Q909VI3 |
| UN number | UN3077 |
| CAS Number | [10058-44-3] |
| 3D model (JSmol) | Fe4(P2O7)3 |
| Beilstein Reference | 3582506 |
| ChEBI | CHEBI:61138 |
| ChEMBL | CHEMBL2104693 |
| ChemSpider | 22943039 |
| DrugBank | DB15835 |
| ECHA InfoCard | 03bb0785-6f27-42b2-a43e-6c32a4c70a0c |
| EC Number | 233-190-0 |
| Gmelin Reference | Gmelin Reference: **15180** |
| KEGG | C18237 |
| MeSH | D017960 |
| PubChem CID | 24857221 |
| RTECS number | TJ8975000 |
| UNII | N3Z6X3JZ0G |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID7020829 |
| Properties | |
| Chemical formula | Fe4(P2O7)3 |
| Molar mass | 745.431 g/mol |
| Appearance | White or grayish-white powder |
| Odor | Odorless |
| Density | 2.9 g/cm3 |
| Solubility in water | Insoluble |
| log P | -17.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.0 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | '30×10⁻⁶ cm³/mol' |
| Refractive index (nD) | 2.1 |
| Dipole moment | 0 D |
| Chemical formula | Fe4(P2O7)3 |
| Molar mass | 745.220 g/mol |
| Appearance | White or grayish-white powder |
| Odor | Odorless |
| Density | 2.87 g/cm3 |
| Solubility in water | Insoluble |
| log P | -17.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.3 |
| Basicity (pKb) | 11.26 |
| Magnetic susceptibility (χ) | +1600.0e-6 cm³/mol |
| Refractive index (nD) | 1.820 |
| Dipole moment | 0 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 146.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2448 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –2448.8 kJ/mol |
| Std molar entropy (S⦵298) | 146.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2677.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2948.6 kJ/mol |
| Pharmacology | |
| ATC code | B03AB09 |
| ATC code | B03AB05 |
| Hazards | |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS07,Warning,H302 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "If medical advice is needed, have product container or label at hand. Keep out of reach of children. Read label before use. |
| NFPA 704 (fire diamond) | 1-0-0-N |
| Lethal dose or concentration | LD50 Oral Rat: >5000 mg/kg |
| LD50 (median dose) | > 6,940 mg/kg (rat, oral) |
| NIOSH | TTJ200 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Iron Pyrophosphate: 1 mg/m³ |
| REL (Recommended) | 45 mg |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-1-N |
| Lethal dose or concentration | LD50 Oral Rat 5,000 mg/kg |
| LD50 (median dose) | > 666 mg/kg (rat, oral) |
| NIOSH | TT4900000 |
| PEL (Permissible) | PEL: 10 mg/m3 |
| REL (Recommended) | 16 mg (as iron) |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Iron(II) phosphate Iron(III) phosphate Ammonium iron(III) pyrophosphate Sodium pyrophosphate Potassium pyrophosphate Calcium pyrophosphate |
| Related compounds |
Iron(II) phosphate Iron(III) phosphate Ferric orthophosphate Ferrous sulfate Sodium pyrophosphate |

