Guanidine Acetic Acid: Time-Tested Chemistry and Modern Relevance
Historical Development
Guanidine acetic acid has roots in early 20th-century European chemical research. The need for novel nitrogen-based compounds pushed early chemists to experiment with simple guanidine structures, eventually leading to the synthesis of guanidine acetic acid. Early medical and biochemical research linked the compound to growth and energy metabolism, connecting it with the agricultural and pharmaceutical revolutions. My own experience with old-school chemistry texts reveals that guanidine derivatives often sit at the edge of new discoveries, not just for specialty chemicals, but for basic metabolic research. As new manufacturing technologies developed, interest in guanidine acetic acid shifted to industrial applications and animal nutrition, expanding its reach into everyday products and feed systems.
Product Overview
This compound appears as a white, crystalline powder with a mild, almost unnoticeable odor. Guanidine acetic acid combines the basic strength of guanidine with the carboxylic punch of acetic acid, making it a polar, water-soluble compound. The industry uses this for more than just feed—its chemical backbone pops up in buffer agents, building blocks for pharmaceutical intermediates, and specialty polymers. Anyone who has worked with animal nutrition or even basic agrochemical synthesis will see the compound’s versatility. Large-scale production relies on demand from both the animal feed industry and the specialty chemical market, showing how fundamental compounds shape multiple sectors without much fanfare.
Physical & Chemical Properties
Guanidine acetic acid usually comes with a melting point around 200°C, stable under normal temperatures, and easy to store when kept dry and away from incompatible substances like strong oxidizers. Chemical structure combines nitrogen-rich guanidine and a carboxyl group, sitting in a stable, slightly hygroscopic crystal form. It dissolves quickly in water, making it handy for both feed mixing and liquid formulations. Employees in production facilities appreciate its relatively low dustiness and straightforward handling, which reduces the kind of safety problems that pop up with more volatile materials. Anyone who’s worked with the stuff knows it won’t break down quickly in standard storage, so stock management gets easier, and product loss from spoilage almost never happens.
Technical Specifications & Labeling
Manufacturers stamp out guanidine acetic acid to meet precise purity standards—99% is common, and certificates of analysis back that up with low impurity levels for substances like heavy metals and residual solvents. Labels must show certification marks, supplier details, expiration dates, and storage instructions. Safety symbols flag the compound as an irritant if you inhale dust or handle without gloves. Feed-grade batches list particle size, recommended inclusion rates, and cross-references to animal nutrition research. In the regulatory world, compliance keeps businesses moving, since unmarked drums or vague testing cause real issues for downstream producers. I’ve seen compliance audits where missing labels or uncertain purity shut down shipments. Reliable suppliers earn reputations not just for chemical quality, but for keeping paperwork and technical specs in order.
Preparation Method
Most industrial plants synthesize guanidine acetic acid by reacting dicyandiamide (DCDA) with chloroacetic acid under aqueous conditions. Catalysts drive the reaction, temperature stays around 90-100°C, and stainless steel reactors become standard to avoid leaching. Crystallization and filtration steps pull the product out; quick cooling and careful separation affect yield and purity. Years of process improvement have shaved energy costs and increased yield to over 90%. Several firms now use continuous synthesis to boost efficiency. For those who’ve worked a production line, controlling pH and temperature means fewer byproducts and easier purification. Side reactions always threaten to spike impurity levels, especially with old equipment or lax maintenance routines, so technical teams put a lot of focus on process monitoring.
Chemical Reactions & Modifications
Guanidine acetic acid provides a useful starting point for chemical modification. Its free amino group and carboxylic acid function allow for peptide coupling, salt formation, and even cyclization to heterocycles. In hands-on lab work, I’ve seen how chemists use guanidine acetic acid to make nutraceuticals, pharmaceuticals, and fine chemicals. Reductive amination extends the molecule, while esterification tunes its solubility for more controlled delivery. Many researchers like to try phosphorylation and acylation, leading to derivatives with improved biological effects. Each modification brings new analytical work—chromatography and mass spec ensure you’re not just making more impurities. The molecule’s flexibility keeps researchers coming back and opens doors to new patent applications.
Synonyms & Product Names
A search across supplier catalogs shows a dozen commercial names: Glycocyamine and Guanidinoacetic Acid pop up the most. In some animal feed circles, it may show as GAA. International chemical databases list it under CAS number 352-97-6. Some distributors try to brand it for specialty use, but most industries stick with generic naming to keep purchasing simple. In practical terms, confusion only happens when new suppliers don’t match up CAS numbers with labeling or when paperwork moves through different languages. My own experience ordering chemical standards reinforces the need to double-check both generic and trade names on purchase orders if you want to avoid mistakes.
Safety & Operational Standards
Factories keep guanidine acetic acid in sealed drums with dedicated dust extraction at handling stations. Most spills clean up easily with water, but direct skin or eye contact needs fast first-aid. Companies enforce PPE—gloves, goggles, dust masks—much like any solid feed additive. Occupational exposure limits aren’t as strict here as with known hazardous compounds, but long-term workers respect the molecule’s basic irritation risk. Internal safety trainings stick with simple rules: no open bags, regular housekeeping, and immediate reporting of any spills or exposures. Regulatory bodies, especially in Europe and Asia, flag improper disposal; wastewater requires basic neutralization before anyone thinks about discharge. Years of on-site inspections drilled into me the need for strong housekeeping—one ignored dust cloud can shut down an entire mixing line or set off a regulatory headache.
Application Area
Most of guanidine acetic acid produced today ends up in animal feed, where it acts as a nutritional supplement that supports creatine synthesis in livestock. Feed companies target pigs, poultry, and aquaculture species, banking on research that shows faster growth and better feed conversion rates. Some niche applications land in pharmaceutical synthesis, lab reagents, and a few personal care brands. Feed mills care about bulk blending ease, nutritional compatibility, and no off-flavors—long experience tells them which ingredients blend well and why customers stick with brands that deliver consistent results. Practical trials by agricultural schools regularly confirm improvements in muscle gain with safe dosage levels. Research into human performance and energy metabolism keeps interest alive in sports nutrition, although regulatory pathways there remain slow and pricey.
Research & Development
New R&D projects in guanidine acetic acid stretch across animal science, biochemistry, and even environmental monitoring. Feed efficiency research dominates, with universities and agricultural extension programs measuring growth benefits in terms of kilograms rather than abstract scores. Lab work connects the molecule to beefed-up creatine pathways, while some pharmaceutical teams test new derivatives for improved solubility and absorption. At any research conference, there’s a clear divide: product managers want real-world feed trial data, and technical scientists focus on metabolic mapping and analytical purity. Even a few startup companies look for applications in sustainable protein production, aiming to reduce greenhouse gas emissions by helping animals convert feed to muscle with less waste.
Toxicity Research
Toxicologists keep a wary but practical eye on guanidine acetic acid, reviewing acute and chronic studies in animals. Most short-term studies find little cause for alarm at doses used in standard feed supplementation, though overdosing or long-term mismanagement can create metabolic imbalances—extra vigilance protects both animal welfare and end-product safety. Experienced nutritionists warn against overuse beyond tested inclusion rates, because pushing too hard on any feed ingredient can tip balances and create new health problems. Human exposure studies remain limited, but workplace safety protocols handle the expected risks. Regulatory reviews in Asia and the EU keep updating inclusion rates and testing guidelines as newer studies refine our understanding of systemic effects and metabolite safety.
Future Prospects
Looking at future trends, guanidine acetic acid sits poised for more innovation. The continuing push for efficient protein production, both in traditional agriculture and new biomanufacturing setups, gives the compound steady market power. More fine-tuned feeding programs focus on both productivity and environmental impact, and guanidine acetic acid’s proven role in improving feed conversion means it won’t fall out of favor soon. Technical teams in Asia and the Americas hunt for even safer, purer forms, backed by better analytics and automated plant controls. Demand for sustainable sourcing links back to how factories manage waste and energy. At the same time, the door remains open for new modifications and medical uses, provided regulatory pathways clear the way. Anyone tracking the intersection of science and agriculture sees that guanidine acetic acid, once limited to the chemical notebooks of early researchers, now anchors wide-reaching programs for both food and health.
What is Guanidine Acetic Acid used for?
Fueling Muscle Growth in Animals
Guanidine Acetic Acid often pops up in conversations about animal feed and performance nutrition. Farmers have searched for ways to keep animals healthy, strong, and productive, especially as demand for meat and dairy grows. To meet that challenge, they have turned to science-backed feed additives. Guanidine Acetic Acid fits right into that quest. It acts as a precursor to creatine. Animals that can make more creatine tend to grow faster. Studies published in respected journals—like Poultry Science and the Journal of Animal Physiology and Animal Nutrition—show its impact on weight gain and muscle development in livestock, especially pigs and poultry. In straightforward terms, it helps animals convert more feed into lean meat instead of fat. For producers aiming to improve profits and lower feed costs, that makes a difference.
Supporting Metabolism
Raising livestock calls for attention to animal health on the cellular level. Guanidine Acetic Acid enters the scene by knocking on the door of energy metabolism. It feeds into the pathway that ends with creatine, a molecule responsible for shuttling energy wherever muscles need it most—during growth and exercise. As a result, animals fed diets containing guanidine acetic acid show fewer signs of fatigue and stress under heavy workloads or summer heat. They bounce back faster, which keeps productivity high and animal welfare standards strong.
Digestion and Feed Conversion
Anyone involved in farming will tell you how important feed conversion is. If animals waste energy digesting poor-quality feed, it costs time and money. Guanidine Acetic Acid gets credit for improving what scientists call nutrient digestibility. Swine and poultry producers report that livestock on supplemented diets show improved protein synthesis and nitrogen retention. That doesn’t just help animals pack on more muscle—it also limits ammonia emissions from manure, helping farms stay within local environmental guidelines.
Regulations and Safe Use
Safety always stands in the spotlight when chemicals mix with food chains. Regulators in the EU, China, and other major agricultural regions have thoroughly reviewed guanidine acetic acid for animal feed. Scientific panels give a green light for use at recommended levels, as long as producers stick to safety data sheets and avoid overdosing. Feed makers remain alert for quality control, knowing that impurities or contamination could affect both animal health and public trust.
Looking Toward More Sustainable Farming
The future of food involves reducing waste, using fewer natural resources, and finding better ways to deliver nutrition. Guanidine acetic acid supports that vision. Fewer resources go into raising strong animals. Environmental footprints shrink, since the compound helps reduce nitrogen emissions from manure. My own conversations with veterinarians and farm advisers reveal a common theme: tools that improve efficiency get a warm welcome. Guanidine Acetic Acid isn’t a cure-all, but it gives producers an extra way to meet growing global demand for animal protein while protecting the planet.
Exploring Further Applications
Curiosity drives research beyond the feed trough. Some experts explore guanidine acetic acid’s potential in aquaculture. Fish farms, like their land-based counterparts, deal with stress, rapid growth goals, and the need to keep water clean. Industry newsletters mention trials with guanidine acetic acid for shrimp and tilapia, reporting stronger growth and disease resistance. Well-designed field trials and transparent data sharing should show where it works best and how it fits into industry standards.
Solving Challenges
Science rarely stands still. Nutritionists and chemists continue to monitor its effects, aiming to fine-tune dosages and combine it with other supplements for even better results. Some groups hope to boost sustainability, make the supply chain more transparent, and clamp down on irresponsible use. The big goal remains clear: keep animals healthy, use resources wisely, and keep food safe for everyone.
Is Guanidine Acetic Acid safe for animal feed?
Understanding Guanidine Acetic Acid’s Role in Feed
Guanidine acetic acid isn’t a household name. On the farm, though, new feed additives show up every year, promising better results and tighter margins. Guanidine acetic acid, sometimes called GAA, has been getting attention for its role in supporting growth, especially in pigs and poultry. The compound serves as a precursor for creatine—an important molecule for muscle development and energy supply. Livestock producers want stronger gains, better feed efficiency, and fewer losses. So, interest in GAA has grown fast since the earliest studies from Europe and China showed improvements when animals ate rations containing this molecule.
Research into Benefits and Risks
Researchers suggest GAA works by improving the creatine supply in muscle tissue, which can unlock growth potential, especially for young animals without easy access to raw creatine. Results from several trials point to increased daily weight gain and improved feed conversion in broilers and piglets. Data shows that GAA increases phosphorus retention, which can help lower phosphorus output in manure. Less runoff means fewer environmental headaches for farmers. Studies published in peer-reviewed journals like the Journal of Animal Science support these findings, giving credibility based on both transparency and reproducibility.
Safety Concerns and Long-Term Effects
Whenever something new comes along, safety questions follow—and rightly so. Feed needs to provide predictable results without causing harm to animals or ending up in the food chain in unsafe concentrations. Excessive exposure to certain feed additives sometimes brings unintended effects like metabolic imbalances, shifts in meat quality, or residues detectable in the final animal product. Fortunately, the safety profile of GAA looks positive at recommended levels. The European Food Safety Authority (EFSA) reviewed the use of GAA as a feed additive and concluded it is safe for pigs and poultry when used as directed. The literature describes no significant toxicological risks or alarming residue levels in meat, eggs, or milk.
That said, limited long-term data remains a challenge. Animal nutrition isn’t just about short-term gains. The feed industry and regulators often call for trials that take into account different breeds, ages, and rearing conditions. Some gaps remain, especially around chronic exposure over several production cycles or interactions with other dietary components. The current consensus among animal nutritionists is based on thousands of animals over dozens of well-conducted studies rather than hunches or short-term observations.
Looking Out for the Whole System
Animal nutrition goes far beyond growth rates. Responsible producers look at gut health, immune function, welfare, and the potential for antimicrobial resistance or toxic byproducts. After using feed supplements on my own small pig operation, I can say that adoption isn’t just about the promise of an extra kilogram per animal. Responsible stewardship means keeping up with evolving research, sticking to recommended doses, and not treating any additive like a silver bullet. Good records, careful observation, and honest conversations with feed reps and veterinarians go a long way toward making the right call for the herd or flock.
Balancing Innovation and Caution
GAA looks promising as part of the toolbox for increasing productivity in animal agriculture. Still, no product replaces solid farming practices, high-quality feed, clean water, and low-stress handling. Pushing beyond recommended applications, blending random supplements, or skipping professional advice creates unnecessary risks. If the science continues to stand up, more producers can use GAA with confidence—always keeping animal wellbeing and food safety at the center of every decision made on the farm.
What is the recommended dosage of Guanidine Acetic Acid?
What People are Asking About Guanidine Acetic Acid Dosage
Questions about the right amount of guanidine acetic acid reflect a real need for better guidance in sports nutrition and livestock supplementation. Guanidine acetic acid, sometimes called guanidinoacetic acid, plays a part in energy metabolism. People try it for boosting athletic performance, building muscle, or improving animal growth in agriculture. Sorting through mixed information online can be confusing, and some recommendations flat-out contradict each other.
What Research Tells Us
Scientific studies run in both humans and animals, but much of the strongest research has focused on animal feed. In pigs and poultry, researchers tracked benefits like improved weight gain and better feed conversion with daily doses ranging from 0.5 to 1.0 grams per kilogram of feed. Excessive amounts don’t add benefits and can sometimes upset metabolic balance. For humans, published studies are less common, and most supplement companies stick with doses in the range of 1 to 3 grams per day, delivered either as tablets or powder.
Dr. Jürgen Scharhag and his research team in Germany explored the impact of guanidine acetic acid on athletes and found that doses near 3 grams per day over four weeks did raise tissue creatine levels, helping support muscle energy. Smaller doses just didn’t get the job done. Doses significantly higher than 3 grams raise concern, since people begin pushing into an unknown territory in terms of safety and side effects.
Safety: A Key Issue With Guanidine Acetic Acid
Nobody should mess around with unproven substances without thinking about long-term risks. Guanidine acetic acid seems okay in the short run at recommended doses, based on animal studies and a handful of clinical trials. High doses can spike levels of methyl groups in the body, putting stress on methylation pathways and possibly bumping homocysteine, an amino acid tied to heart disease. People who already struggle with heart health or vitamin B deficiencies have more reason to talk to a health professional first.
Why Quality and Individual Differences Matter
Like any supplement, quality varies. Not every manufacturer tests rigorously. I once tried a bargain supplement from a no-name brand, and its powder clumped in the container. That experience killed my trust. Third-party testing, GMP certifications, and transparency about ingredients build consumer trust.
Each body processes amino acid derivatives differently. Teen athletes, middle-aged bodybuilders, older adults—everyone absorbs and reacts to supplements based on their diet, genetics, health, and activity level. Liver and kidney function make a difference too. This isn’t a "one size fits all" world, so sticking to the lower end of the dose range when starting makes sense.
Practical Steps and Solutions
Before adding guanidine acetic acid to a daily routine, a discussion with a registered dietitian or physician pays off. Ask questions about interactions with medications and existing health conditions. Quality supplements demand up-to-date COAs (certificates of analysis). Avoid chasing after mega-doses.
If industry leaders and supplement brands wanted to help, they’d fund bigger, longer-term studies in humans. Clear guidelines would help prevent misuse. All of us benefit when supplement facts reflect real-world experience and published data, not just marketing rhetoric. Until then, starting at a dose around 1 to 3 grams per day, watching for side effects, and treating supplements like any other tool—useful, but never a miracle—offers the most practical way forward.
How should Guanidine Acetic Acid be stored?
Getting Real About the Risks
Guanidine acetic acid isn’t your everyday pantry staple. This chemical often crops up in laboratories and the animal feed trade. At work, I’ve run into this stuff on lab benches and in bulk bags at agricultural warehouses. What stands out every time: mistakes with chemical storage carry real consequences. I'm talking about property damage, health scares, or, worst of all, folks getting hurt. The material itself can irritate eyes, skin, and the respiratory tract, so leaving things to chance is reckless.
Storage Starts with Dryness
Moisture kills here, plain and simple. Guanidine acetic acid absorbs water in the air. Water in the container can lead to clumping or unwanted reactions. I once watched a warehouse crew scoop hardened chunks out of a drum because they’d stored it on a wet floor without a proper seal. Shelf life tanked. So, keep storage areas bone dry. If humidity climbs in your climate, desiccant packs and air-tight seals can save a lot of trouble — and money.
Temperature Matters
Nothing radical is needed, but wild swings in temperature shorten the life of a lot of chemicals, this one included. Fluctuations also make labeling unreadable as condensation and heat blur ink or glue labels. At one livestock supply outlet, the team kept pallets of additives next to an uninsulated metal wall. In July, that gear felt almost boiling. Fumes snuck out. On a crisp February morning, the powder clumped. Aim for a stable room temperature, away from heat sources. No need for a fridge—just stay away from stifling warehouses or freezing sheds.
Seal It Up, Keep it Separate
Tossing chemical bags into mixed storage leads to confusion and potential disaster. Guanidine acetic acid powders pick up flavors and, more importantly, can react with strong oxidizers or acids stored nearby. Anyone who’s ever seen a spill spread through mismatched supplies knows why this is bad news. I always stick to dedicated shelving or pallets, well away from anything it might react with. If unloading lots of different products from a single truck, organize them before they ever hit the shelf.
Labeling and Safety Gear Are Non-Negotiable
People grab the wrong container. It happens. Clear, solvent-proof labels—printed, not handwritten—save nerves and prevent accidents. On seeing torn bags and marker labels wiped clean by spilled water, I knew someone would make a mistake. Always include the product name and hazard symbols, so new hires or folks on overtime shifts stay on track. At every job, I see gloves, goggles, and long sleeves as minimum standards. Rushing or skipping gear over “just a minute” is courting disaster.
Small Steps Go a Long Way
Guanidine acetic acid doesn’t demand fancy climate control or high-tech containment. Clean, dry shelves, a stable room temperature, clear labels, and simple protective gear do the work. This isn’t about playing chemistry cop; it’s about respecting a material that works hard in industry and agriculture but bites back if handled carelessly. With just a few basic measures, costly messes and emergencies drop out of the picture.
What are the benefits of using Guanidine Acetic Acid in livestock nutrition?
A Breakthrough for Growth and Muscle Building
Feeding animals for rapid growth and muscle mass has always been a tricky business. Cattle, poultry, and swine producers pay close attention to feed formulas, searching for that extra edge. Over the years, I’ve watched barns put high hopes in protein, amino acids, probiotics, and yet, sometimes something newer provides a better return. Guanidine Acetic Acid is exactly that sort of development in animal nutrition — it goes right into the building blocks, helping animals build muscle efficiently.
Guanidine Acetic Acid steps in as a precursor to creatine, a nutrient the body turns to for energy. In humans, creatine means power for athletes, and research shows animals get a similar boost. Chickens, pigs, and cattle all need a lot of energy to pack on lean muscle, and they can’t always make enough creatine from feed ingredients alone. With extra input through feed, Guanidine Acetic Acid helps fill those gaps.
Feed Efficiency and Sustainability
Feed bills can make or break a farm. Adding Guanidine Acetic Acid means animals extract more value from every mouthful. Trials on broilers and pigs show better weight gain with no extra feed. For chicken growers, this means more breast meat without wasting protein. For pig producers, it translates to better carcass quality and less fat — not only higher sale value but a quicker turnaround from farrowing to market.
Livestock farming faces pressure to produce more with less — less land, less grain, less output of greenhouse gases. Guanidine Acetic Acid fits into this bigger picture by improving feed conversion rates. Animals that grow faster with less grain mean fewer resources go into the same output. Research backs up lower nitrogen excretion in manure, which reduces nutrient runoff and environmental impact. For producers, that's a win, meeting both economics and new regulatory standards.
Health and Stress Tolerance
Livestock deals with stressful conditions. Summer heat, crowded barns, long transport days — these all sap energy and put animals at risk for poor weight gain or illness. Guanidine Acetic Acid supports intracellular energy during these times. Animals on feed containing this supplement maintain performance during challenges, avoiding the dips seen in traditional regimens.
Producers see value in sturdier animals. Sick pigs or chickens mean lost profits, extra veterinary costs, and food safety questions for buyers. By giving animals an energy buffer, Guanidine Acetic Acid offers a tool to get through rough periods with fewer setbacks. Field experiences show improvements not just in performance, but in persistency and survivability through high-stress events like weaning and transport.
Research and Real-World Impact
Science keeps offering more on Guanidine Acetic Acid’s role. Peer-reviewed studies in journals like Animal Feed Science and Technology, plus real-world performance records, show better average daily gains and improved feed efficiency. There’s also growing data supporting more favorable meat yields and reduced fat deposition.
Adopting Guanidine Acetic Acid isn’t just about chasing the next supplement. It lines up with producer goals: more product with less input, healthier animals, lighter environmental footprints. From a nutritionist’s desk to the feed mill to the barn floor, this additive keeps earning its place in the feed industry by proving its value time and again.
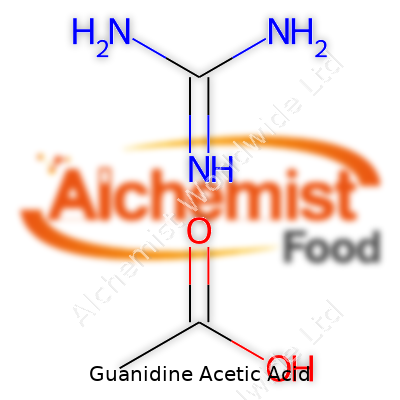
| Names | |
| Preferred IUPAC name | 2-guanidinoacetic acid |
| Other names |
GAA Guanidinoacetic acid N-(Aminomethyl)guanidine Glycocyamine |
| Pronunciation | /ɡwəˈnɪdiːn əˈsiːtɪk ˈæsɪd/ |
| Preferred IUPAC name | 2-guanidinoacetic acid |
| Other names |
Glycineamidinecarboxylic acid GAA N-(Aminoiminomethyl)glycine Amidinoglycine |
| Pronunciation | /ɡwəˈnɪdiːn əˈsiːtɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | [[352-97-6]] |
| Beilstein Reference | 1721165 |
| ChEBI | CHEBI:131786 |
| ChEMBL | CHEMBL1232561 |
| ChemSpider | 63754 |
| DrugBank | DB14520 |
| ECHA InfoCard | 03d8d1e4-362a-4860-bbea-0f83048eae49 |
| EC Number | EC 206-951-6 |
| Gmelin Reference | 67722 |
| KEGG | C01758 |
| MeSH | D015909 |
| PubChem CID | 2724301 |
| RTECS number | MC1406000 |
| UNII | 6HG8UB2MUY |
| UN number | UN2682 |
| CompTox Dashboard (EPA) | EPA CompTox Dashboard (DSSTox) ID: DTXSID5011878 |
| CAS Number | 352-97-6 |
| 3D model (JSmol) | `C(N=C(N)N)CC(=O)O` |
| Beilstein Reference | 1740243 |
| ChEBI | CHEBI:32875 |
| ChEMBL | CHEMBL1230480 |
| ChemSpider | 89213 |
| DrugBank | DB11441 |
| ECHA InfoCard | 24-212-787-1 |
| EC Number | EG-200-347-6 |
| Gmelin Reference | 100222 |
| KEGG | C05158 |
| MeSH | D004362 |
| PubChem CID | 2724274 |
| RTECS number | FD5250000 |
| UNII | PHQ595216G |
| UN number | UN3509 |
| CompTox Dashboard (EPA) | DTXSID5067276 |
| Properties | |
| Chemical formula | C3H7N3O2 |
| Molar mass | 118.11 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.341 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.24 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 12.45 |
| Basicity (pKb) | 11.72 |
| Magnetic susceptibility (χ) | -46.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.525 |
| Viscosity | 10-30 mPa.s (20°C) |
| Dipole moment | 6.35 D |
| Chemical formula | C3H7N3O2 |
| Molar mass | 117.13 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.341 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.24 |
| Acidity (pKa) | pKa1 = 2.4, pKa2 = 7.0, pKa3 = 12.4 |
| Basicity (pKb) | 11.04 |
| Magnetic susceptibility (χ) | -29.3×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.525 |
| Viscosity | 2.39 cP (20°C) |
| Dipole moment | 8.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −302.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -613.5 kcal/mol |
| Std molar entropy (S⦵298) | 188.7 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -451.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -613.7 kJ/mol |
| Pharmacology | |
| ATC code | A16AA16 |
| ATC code | A16AA16 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Lethal dose or concentration | LD50 oral, rat: 2380 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Guanidine Acetic Acid: 2380 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | Not Established |
| REL (Recommended) | Recommended Exposure Limit (REL) for Guanidine Acetic Acid: **10 mg/m³ (as 8-hour TWA)** |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 216.1 °C (closed cup) |
| Autoignition temperature | 460°C |
| Lethal dose or concentration | LD50 (oral, rat): 1790 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,200 mg/kg (rat, oral) |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/m³ |
| Related compounds | |
| Related compounds |
Glycine Arginine Creatine Guanidine Acetic acid |
| Related compounds |
Creatine Guanidine Acetic acid Glycine Arginine Creatinine |

