Fumaric Acid: A Deep Dive into Its Past, Present, and Future
Tracing the Roots: Historical Development
Long before the modern food and chemical industries packed their shelves with fumaric acid, its story began in the quiet corners of nature. European scientists started isolating fumaric acid from fumitory plants in the early 1800s. Names like Carl Wilhelm Scheele often pop up while discussing early organic acid discoveries, and by the mid-nineteenth century, researchers understood how to produce this compound through chemical synthesis rather than tedious extraction from plants. Factory-scale fermentation processes didn't emerge until the twentieth century, when demand for safe, reliable food acidulants forced industry to seek efficient alternatives to tartaric and citric acids. Over time, technologies for producing fumaric acid grew alongside other fermentation-derived chemicals, giving rise to methods that are still refining yields and efficiency decades later.
Product Overview
Fumaric acid looks like a fine, white crystalline powder, calling to mind common kitchen baking ingredients, although it carries a tang that’s sharper than lemon juice. This acid belongs to the family of dicarboxylic acids and finds its way into food processing, pharmaceuticals, animal feed, and even industries molding resins and polyester. Manufacturers prize it because its sour punch won’t mask the natural flavor of foods or beverages and its crystal structure holds up in products that need to last on shelves. Pharmacies blend fumaric acid into certain treatments for skin or autoimmune disorders. Its role stretches further yet, reaching textile, plastics, and agricultural sectors that value a stable, predictable acidulant for technical applications.
Physical and Chemical Properties
This compound’s molecular formula is C4H4O4. Its melting point sits at about 287°C. It dissolves slowly in cold water, speeding up as temperatures climb, and shows almost no solubility in organic solvents like ether. Chemists appreciate its ability to form salts and esters, known as fumarates, which open up a spectrum of uses in food and pharmaceutical science. Fumaric acid’s structure — two carboxyl groups double bonded across a carbon chain — gives it a lower acidity than some dicarboxylic cousins but keeps it stable against spoilage and degradation.
Technical Specifications and Labeling
Quality standards steer production choices, and the food grade fumaric acid on today’s shelves meets FCC (Food Chemicals Codex) and E-number E297 certifications. Testing covers heavy metals, purity above 99.5 percent, and acceptable limits for ash, arsenic, and moisture. Manufacturers print batch codes, expiry dates, and clear usage directions across every drum or bag. Transport and storage guidelines ask for cool, dry spaces and non-reactive packaging, usually polyethylene or paper bags lined with an inner seal. Global codes and registration numbers, such as CAS 110-17-8, help researchers, safety officials, and industry buyers check the identity and compliance of every shipment.
Preparation Methods
Decades spent refining synthesis have nudged industry away from searching plants to batch fermentation using fungi like Rhizopus species. Starting with glucose or other sugars, fungal cultures slowly turn them into fumaric acid over the course of a few days. Once the fermentation runs its course, processors separate the acid from liquid broth using filtration and precipitation before drying and grinding the finished crystals. There’s also an old-school route involving the catalytic isomerization of maleic acid — essentially rearranging similar molecules under heat and catalyst action until they flip into fumaric acid. This chemical process, while efficient, lost ground to biotechnological methods thanks to the hunger for renewable, plant-based feedstocks.
Chemical Reactions and Modifications
Chemists have earned their stripes exploring how fumaric acid reacts in various settings. Heating can drive it into an anhydride form, giving it a slightly different set of properties useful in plastics and synthetic polymers. Formation of salts and esters happens under controlled mixing with alcohols or bases, producing compounds that deliver medicine more efficiently or improve animal feed efficiency. In polymer chemistry, fumaric acid bends nicely into cross-linked networks, which support everything from thermal-resistant plastics to bulk resins. Researchers keep developing subtle modifications that increase solubility, extend shelf life, or change its behavior in specific chemical environments.
Synonyms and Product Names
Markets know this acid by many names: trans-butenedioic acid, allomaleic acid, and acidum fumaricum surface in chemical catalogs. In food and beverage settings, suppliers may label it simply as E297. Some pharmaceutical circles use terms like fumarate for its salts, especially in prescription drugs for chronic illnesses. Trade names rarely stray far from “fumaric acid,” possibly because chemical buyers prefer clarity and precision in labeling and documentation.
Safety Guidelines and Operational Standards
Safety matters every step of the way, from the lab bench to the loading dock. Dust from fumaric acid, if inhaled or allowed to linger on skin, stings and irritates. Factory settings point workers to gloves, goggles, and, when high concentrations of dust threaten, particulate masks. Regulatory bodies set exposure limits and proper handling practices, with clear spill and cleanup protocols that focus on preventing slips or respiratory issues. Food-grade sources pass through audits for allergens, heavy metals, and other contaminants. Modern training around chemical handling and hygiene helps keep process workers safe and reduces risks during transport, storage, and dispensing.
Application Areas
Anyone who’s looked at a powdered drink flavor packet, leavening agent, or sports chew probably came close to a dose of fumaric acid. Its food uses rely on a consistent tartness and a relentless ability to hold up in dry mixes, letting it serve in baking, beverages, candy, and dairy stabilization. Pharmaceuticals see it integrated into long-acting tablets and as part of treatments for conditions like psoriasis and multiple sclerosis, changing how the body’s immune system behaves. Resins, inks, and paints absorb it as a building block for finished products, while agriculture leans on its salt forms to help supplement animal feed.
Research and Development
Modern labs keep chasing ways to pull more value out of fumaric acid production, exploring new fermentation microorganisms, genetically altered fungi, or enzymes that can work faster and cleaner. Current research seeks higher yields, lower byproducts, and easier purification steps—all with sustainability in mind. Scientists are probing how blends with other organic acids might stretch shelf life, alter nutritional outcomes, or lead to biodegradable plastics. Pharmaceutical research uses new derivatives derived from fumaric acid for disorders beyond autoimmune diseases, charting progress with each clinical trial. Cross-disciplinary projects often bring together food chemists, materials scientists, and bioprocess engineers who test whether this overlooked acid can help solve problems far beyond its original industry boundaries.
Toxicity Research
Fumaric acid’s safety record holds up well, but the story isn’t without caution. Animal studies at very high doses indicate gut irritation, and occupational exposure to dust concentrates the risk of skin, eye, and lung irritation. Food safety bodies from the US FDA to the European Food Safety Authority have reviewed evidence for acute and chronic effects, regulating intake and qualifying it as generally safe for intended uses. Medical formulations undergo additional scrutiny based on delivered dose and context. Research efforts continue to monitor possible links to allergies, sensitivities, and rare cases of metabolic disturbance, acknowledging that broad industrial adoption requires ongoing vigilance.
Looking Forward: Future Prospects
Sights turn toward greener chemistry, and fumaric acid stands ready as a bridge between renewable feedstocks and a range of environmentally friendly products. Biotechnologists keep tweaking fermentation strains in search of faster growth or the ability to use non-edible waste streams as inputs. Packaging and polymer researchers consider fumaric acid-based plastics as options for single-use goods, moving toward biodegradability that leaves a lighter mark on the environment. Food scientists tinker with blends and fortification for tailored health benefits, while medical research gives hope for expanding its reach into chronic disease. With every new breakthrough, this humble white powder keeps opening doors far beyond its roots in turn-of-the-century chemistry.
What is fumaric acid used for?
Why I Keep Noticing Fumaric Acid in Ingredient Lists
Fumaric acid doesn’t turn heads. On a label, it looks like any other “acid” – something mysterious, maybe a little off-putting. But over the years, as someone who keeps an eye on what goes into my food and medicine, its name keeps popping up. And the more I dig, the more I realize – this isn’t some fringe industrial additive. Fumaric acid gets mixed into a lot of things most people eat and use every day.
Adds Tang and Freshness in Food
Walk down the snack aisle and you’ll find chips, tortillas, and bakery items relying on fumaric acid. Why? This stuff adds a sharp, tart flavor. No, it doesn’t taste quite like lemon juice, but it gives that mouth-watering bite in sour candies and powdered seasonings. The magic isn’t just taste – it helps foods stay fresh longer. Compared to citric acid, less is required, so companies use it to stretch the shelf life of baked goods without blasting your taste buds with sourness.
Not long ago, I learned that tortillas and flatbreads keep their soft texture because fumaric acid tweaks the pH, slowing down mold and staling. That’s a huge deal for families and businesses trying to avoid tossing out stale food. Toss some powdered lemonade mix into the mix: smokers report it balances out flavors and keeps sweetness from turning cloying.
Supporting Health with Precision
Doctors and pharmacists know fumaric acid for its role in medicine. Decades ago, doctors in Europe tried it for skin conditions like psoriasis. Today, certain fumaric acid esters serve as active ingredients in treatments for psoriasis and multiple sclerosis. The scientific backing isn’t flimsy—these derivatives help modulate the immune system, reducing inflammation and flare-ups. While patients should never take regular fumaric acid powder themselves, the medicine cabinet shows just how versatile one chemical can be.
A Quiet Workhorse in Industry
Anyone curious about what holds plastics together or keeps resin strong knows acids don’t just belong in kitchens. Fumaric acid steps up in the manufacturing of resins, coatings, and paints. Builders appreciate how it hardens and cross-links plastic or fiberglass, keeping home structures tough and durable. Pharmacies use it to control acidity in pills or capsules. Even animal feed manufacturers rely on it—livestock stays healthier when their feed acidifies, crowding out harmful microbes. Here, safety matters. Regulators such as the FDA and EFSA set strict rules for how much gets used, so people and animals don’t face extra risks.
What Responsible Use Looks Like
Not all chemicals deserve a spot in our food or homes. Fumaric acid earns its place because it passes the safety tests experts demand. Still, no industry should use more than needed, and consumers should check those ingredient panels. That means reading up, looking for food-grade certifications, and double-checking if you’re prone to allergies. Science hasn’t linked fumaric acid with chronic health threats at approved levels, but balance matters. Anyone with health issues—say, chronic skin or autoimmune problems—should talk to a doctor before jumping on supplements or new foods.
Building Trust by Staying Transparent
Transparency keeps this conversation healthy. Every company adding fumaric acid needs to explain why and how much they use. Regulators must keep up with new studies and products to ensure nothing gets overlooked. Industry players can lead by funding research and sharing results. As a shopper and writer, I want straight answers—both for my own peace of mind and for the sake of my family’s health.
Is fumaric acid safe to consume?
What is Fumaric Acid Doing in Our Food?
Most people spot strange names on food labels, then brush right past them. Fumaric acid shows up in lots of baked goods, drink mixes, and processed foods. Food scientists use it to give products a sour kick, preserve things longer, and help dough rise. It occurs naturally in fruits and fungi, but the food industry usually creates it in factories using chemical reactions and certain types of fungi.
Science Backs Up the Safety—Within Limits
Food regulators in the United States, Europe, and other places describe fumaric acid as safe for eating. The U.S. Food and Drug Administration (FDA) has labeled it “Generally Recognized As Safe” (GRAS) at levels found in food. Europe assigns it the number E297 on ingredient lists. Large health groups have studied this compound for decades, finding no major problems for most people at typical food levels. People need to eat over three grams per kilogram of body weight at once before seeing toxic effects, based on research—an amount much higher than anyone manages to eat in real life through food.
Regulatory agencies look for side effects, cancer risks, impacts on organs, and long-term problems before approving additives. Fumaric acid passes these tests. The real risk sits with consuming way beyond recommended amounts, or if someone already deals with sensitive stomach issues. Excess can lead to abdominal pain or cramps. Doctors have even used fumaric acid derivatives to treat some medical conditions, like psoriasis, showing there is some flexibility in how the compound gets used and studied.
Experience at the Dinner Table
My family lives in a busy suburb, just like millions of others. Schedules get tight, and sometimes quick packaged foods save us from skipping dinner entirely. I often check ingredients. Seeing fumaric acid pop up in tortillas or fruit bars makes me check my sources. After reading what scientists and health officials say, I’m not sweating about an occasional snack containing it. I’d feel more worried about the sodium, sugars, and trans fats in most processed products—fumaric acid seems low down the danger list based on current data.
No Reason to Panic, But Thinking About the Big Picture Makes Sense
Trust in food additives depends a lot on how much people eat. Eating a varied diet full of fruits, veggies, proteins, and grains already brings a host of acids, vitamins, and minerals. Relying too much on packaged or highly processed foods could mean getting more additives—including fumaric acid—than people realize. For those with stomach problems or those who react to citrus fruits, avoiding foods high in acids, including fumaric, makes sense. Everyone else can feel okay enjoying that piece of sour candy or slice of supermarket bread from time to time.
The conversation should focus on healthy eating habits overall, not villainizing a single ingredient. Reducing processed foods helps lower exposure to all sorts of additives, not just fumaric acid. Companies could be more transparent about how their additives get made and studied, so the public can make informed choices. Eating more actual foods over packaged goods remains the safest bet—something health experts agree on everywhere.
What are the benefits of fumaric acid?
Fumaric Acid: Not Just a Food Additive
Fumaric acid pops up in ingredient lists more often than most people realize. It often hides in plain sight, showing up in baked snacks, processed foods, and even health supplements. Growing up, I never paid much attention to what was in my favorite candies or tortillas, but these days, I've started noticing just how many familiar products rely on this simple compound.
Why Manufacturers Use It
Bakers and snack companies count on fumaric acid for its sour punch and dependable shelf life. In tortillas, for example, it helps keep the bread soft for longer stretches without losing flavor or freshness. Regular flour tortillas used to stiffen up quickly if left out too long, but with this acid, days-old wraps stay flexible. Fumaric acid doesn’t just add tartness—its solid structure resists humidity, which slows down mold and spoilage. Back in my college days, I’d grab tortillas and chips at the start of the week. The packs that listed fumaric acid didn’t mold as quickly as the plain ones, especially through the summer months.
Supporting Digestive Health
Some supplements include fumaric acid for its effects on digestion. The body already produces it naturally, converting it as part of the Krebs cycle—one of the ways we extract energy from food at a cellular level. People often overlook how little changes in diet or supplementation can nudge overall metabolism in the right direction. Research even explores how it could help folks with psoriasis or multiple sclerosis because of its ties to cellular regulation and immune support. That doesn’t mean it’s a miracle cure, but early studies show that certain fumarate compounds, such as those in prescription medications, help manage inflammation.
Better Beverages and Candy
Soft drinks and candy makers reach for fumaric acid for good reason. Its tang holds up under heat and doesn't dissolve as fast as citric acid. That slow dissolve works great in sour candies, letting the taste hang around longer on the tongue. As a kid, I’d marvel at how some sweets seemed to pack a stronger punch, and today's ingredient labels reveal fumaric acid as the secret weapon. In fruit-flavored sodas, it keeps flavors bright without overwhelming the batch with excess sweetness. The same trick gives sports drinks and some vitamin C tablets their familiar zip.
Environmental and Practical Benefits
Fumaric acid comes from both traditional and greener production methods, including fermentation—which echoes trends in sustainable food science. Modern fermentation allows factories to generate the acid with fewer fossil fuel byproducts, creating less pollution. So, manufacturers shave off costs and carbon emissions at the same time. There’s real value in everyday chemistry like this, especially as shoppers look for products that do less harm to the planet.
What to Watch For
No ingredient suits every person all the time. Some folks have food sensitivities; a handful of people may notice stomach upset if they eat lots of processed foods with food acids, including fumaric acid. As with anything in daily nutrition, moderation helps. Anyone with specific concerns can check for E297 on packaging or talk with a dietitian. But for most people, it works behind the scenes to keep groceries tasting fresher and better for longer.
Are there any side effects of fumaric acid?
Why People Care About Additives in Food
Walking through grocery aisles, you’re bound to find unfamiliar ingredients tucked away on food labels. One of those names is fumaric acid. It pops up often in baked goods, drink mixes, and even some candies. The sour taste in some tart treats traces back to this compound. It preserves, flavors, and keeps food fresh.
I remember asking myself about these long chemical names while shopping. Should I worry? Are they just food science doing its job, or do they bring hidden risks?
What We Know About Fumaric Acid
This additive gets produced in big factories from fermented sugar or sometimes through a chemical reaction with maleic anhydride. Fumaric acid shows up naturally in fruits and vegetables—think mushrooms or lichen. The amount used in food skies below what you’d find in medicine or skin creams.
The U.S. Food and Drug Administration puts fumaric acid on its list of “Generally Recognized as Safe” substances for eating. The European Food Safety Authority also reviewed studies and didn't find alarming red flags for regular eaters. In my own search through medical papers and food safety reports, I found that experts don’t link common food levels of fumaric acid to major health threats for most people.
Possible Side Effects and Who Feels Them
Diving into details, most healthy folks handle the small doses added to snacks and drinks without a problem. Eating food with a sprinkle of fumaric acid now and then doesn’t lead to widespread complaints in the general public. Some people, though, do react. Some case reports and clinical trials reveal side effects, mostly after large doses:
- Stomach upset: Some experience mild abdominal pain, nausea, or diarrhea when they eat or drink something with a high acidic punch—far above what’s typical in food.
- Allergic reactions: Rarely, people sensitive to food acids could face itchy skin or rashes, but this problem looks extremely uncommon.
- Kidney trouble: There’s worry for folks with chronic kidney disease since acid builds up more easily in their system. Doctors usually flag this for people using fumaric acid for medical conditions rather than diet, but caution still matters.
If you use medication containing fumaric acid to treat health conditions—like psoriasis or multiple sclerosis—the story changes. Medical doses come much larger, where side effects get serious. There, experts track blood work for kidney issues and monitor for low white blood cell counts or liver injury. These cases stick out in pharmaceutical literature, not in grocery stores.
Sorting Out Risks and Solutions
No food ingredient should live above scrutiny. Sensible steps keep everyone safe. Watchdog agencies keep checking for new evidence. Consumer advocacy groups play a role, too, by pushing for clean and clear labels. If anyone feels weird after eating a food with this ingredient—especially if allergies or kidney disease run in the family—talking with a healthcare professional helps figure out what’s going on. Checking batch numbers or saving packaging sometimes tracks down the cause.
To keep food healthy and enjoyable, producers can lower acid levels whenever possible, try alternative flavors, use natural sources, and conduct more independent research. When buyers press for transparency, companies have more incentive to make safer choices. Reading labels adds a layer of control for anyone wanting to avoid added acids, and raising awareness helps people make informed decisions.
Is fumaric acid natural or synthetic?
Where Fumaric Acid Comes From
A lot of people see "fumaric acid" on an ingredient list and wonder what’s going into their food. Fumaric acid has two main sources: it exists naturally in certain fruits and vegetables, but most of what ends up in processed food gets made in a factory.
Some fruits—apples, mushrooms, and lichen—carry small amounts of fumaric acid. Our own bodies make tiny amounts during the Krebs cycle, that energy-producing process biology teachers love to mention. Despite these natural connections, companies rarely gather fumaric acid by squeezing fruit. They make it out of necessity, using industrial processes for cost, purity, and scale.
Why the Synthetic Route Wins
Nobody’s turning fields of apples into enough fumaric acid to meet global food or supplement demand. It’s true, biotechnology can coax certain molds, like Rhizopus species, into making fumaric acid. Still, the dominant approach relies on good old chemistry—specifically, transforming maleic anhydride, itself a byproduct of oil refining, into fumaric acid.
Factory production guarantees consistent results. Every batch comes out with the same properties. This kind of regularity helps keep manufacturing lines running smoothly and food quality stable, from your favorite loaf of bread to tart candies. Few natural sources can offer these benefits at such a low cost.
Safety and Health Considerations
I’ve seen plenty of confusion about whether “synthetic” automatically means unsafe. That’s not always the case, and fumaric acid is a good example. The Food and Drug Administration labels it “Generally Recognized as Safe” (GRAS) when used as a food additive. It’s been part of the food world for decades. Its main job: adding a sour taste, preserving shelf life by keeping out mold, and adjusting acidity.
Overuse of any food additive raises concerns. Large amounts of fumaric acid could disturb digestion in some people, especially those with sensitive guts. Still, typical use levels remain much lower than what might cause irritation.
Food Trends and Natural Claims
A lot of brands advertise products as “all natural” and fuel skepticism about anything synthetic. Yet, “natural” on a label doesn’t guarantee safety, purity, or health. Snake venom, for example, is natural. The debate about natural versus synthetic has as much to do with public perception as science. People want to trust the food they buy—and that trust often hangs on the word “natural.”
I grew up in a home where we cooked from scratch, and grandma’s pantry rarely carried products with unfamiliar additives. These days, reading labels takes more detective work. Many shoppers pick based on a sense that natural is better, even if there’s little evidence it matters in the case of food acids. Scientific studies, including those published by independent journals and reviewed by food safety experts, underline that the source (natural or synthetic) often makes less of a difference to health than proper use and overall diet.
Realistic Solutions for Clearer Food Choices
Food companies and regulators could do a better job explaining where ingredients come from, why they’re used, and how much is present. Greater transparency earns trust. Ingredient lists might be clearer about whether additives are derived from plants, microbes, or petroleum-based sources.
More science-backed education in schools on reading labels, not just memorizing chemical names, would help future consumers make decisions rooted in fact. As food production grows ever more complex, real understanding will rely less on buzzwords like “natural” and more on genuine, evidence-based information about what enters our bodies.
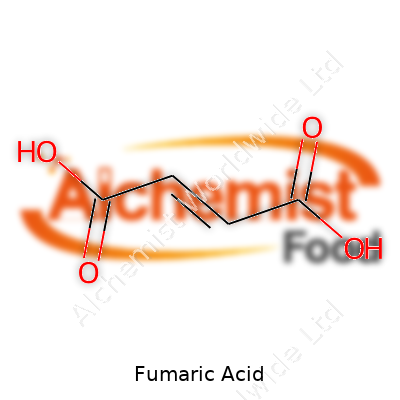
| Names | |
| Preferred IUPAC name | (E)-but-2-enedioic acid |
| Other names |
trans-Butenedioic acid Boletic acid Lichenic acid |
| Pronunciation | /ˈfjuːmərɪk ˈæsɪd/ |
| Preferred IUPAC name | (E)-but-2-enedioic acid |
| Other names |
Boletic acid Lozenge acid Lichenic acid Allomaleic acid Donitic acid |
| Pronunciation | /ˈfjuː.mær.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 110-17-8 |
| 3D model (JSmol) | `/assets/jmol-3d/fumaric-acid.cml` |
| Beilstein Reference | 1718734 |
| ChEBI | CHEBI:18012 |
| ChEMBL | CHEMBL1349 |
| ChemSpider | 10208 |
| DrugBank | DB03181 |
| ECHA InfoCard | EC Number: 203-743-0 |
| EC Number | 2.3.1.2 |
| Gmelin Reference | 60492 |
| KEGG | C00122 |
| MeSH | Fumaric Acid |
| PubChem CID | 444972 |
| RTECS number | LU5950000 |
| UNII | K8FD500UOQ |
| UN number | UN9072 |
| CAS Number | 110-17-8 |
| 3D model (JSmol) | `"Fumaric Acid"3D model (JSmol) string: C1=CC(=O)OC(=O)C1` |
| Beilstein Reference | 1208170 |
| ChEBI | CHEBI:18012 |
| ChEMBL | CHEMBL1347 |
| ChemSpider | 10213 |
| DrugBank | DB03181 |
| ECHA InfoCard | 100.013.951 |
| EC Number | EC 203-743-0 |
| Gmelin Reference | 607348 |
| KEGG | C00122 |
| MeSH | D005652 |
| PubChem CID | 444972 |
| RTECS number | WS3850000 |
| UNII | TX16XOL3WL |
| UN number | UN9126 |
| Properties | |
| Chemical formula | C4H4O4 |
| Molar mass | 116.07 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.635 g/cm³ |
| Solubility in water | 6.6 g/L (20 °C) |
| log P | -1.37 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.03, 4.44 |
| Basicity (pKb) | 1.48 |
| Magnetic susceptibility (χ) | -47.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.508 |
| Dipole moment | 2.69 D |
| Chemical formula | C4H4O4 |
| Molar mass | 116.07 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.635 g/cm³ |
| Solubility in water | 6.6 g/L (20 °C) |
| log P | -1.17 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.03, 4.44 |
| Basicity (pKb) | 1.90 |
| Magnetic susceptibility (χ) | -45.2e-6 cm³/mol |
| Refractive index (nD) | 1.508 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 153.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -796.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1336 kJ/mol |
| Std molar entropy (S⦵298) | 153.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -819.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1336 kJ/mol |
| Pharmacology | |
| ATC code | A07XA03 |
| ATC code | A51AA02 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 307°C |
| Autoignition temperature | 540 °C (1004 °F; 813 K) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 oral rat 9300 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat) 9300 mg/kg |
| NIOSH | KW2975000 |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 0.003 mg/kg bw |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | ["GHS07", "GHS05"] |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | Wash … thoroughly after handling. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 230°C |
| Autoignition temperature | 360 °C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Rat oral 9300 mg/kg |
| LD50 (median dose) | LD50 (median dose): 10700 mg/kg (oral, rat) |
| NIOSH | RN8225000 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 800 mg |
| Related compounds | |
| Related compounds |
Maleic acid Malic acid Succinic acid Tartaric acid Phthalic acid |
| Related compounds |
Maleic acid Malic acid Succinic acid Tartaric acid Citric acid |

