Ferrous Sulfate Monohydrate: A Closer Look at an Old Staple with New Relevance
Historical Development
Ferrous sulfate monohydrate traces a story that goes back centuries. People first started using iron vitriol, an earlier form of ferrous sulfate, during the Roman era for dyeing textiles and as a component in ink. By the 19th century, chemists figured out ways to extract and purify ferrous sulfate more efficiently, allowing it to find a place not only in fabric and ink production but also in agriculture and medicine. In recent decades, it’s become better known as an iron supplement and a soil amendment for crops. Changes over the years in manufacturing, driven by both technology and environmental law, have eliminated a lot of impurities that used to be common in the product. These days, quality standards land far higher, and the purity level now expected would have sounded outlandish fifty years ago.
Product Overview
Ferrous sulfate monohydrate shows up frequently as a greenish-blue or pale blue powder or crystal. It’s the go-to iron source for feed additives, pharmaceuticals, and fertilizer because of its high bioavailability and relatively simple handling. In my own work supporting agricultural clients, I’ve seen ferrous sulfate help restore iron-deficient soils—yields jump, and crops regain their color and vigor. Unlike elemental iron, ferrous sulfate dissolves more readily in water, which makes application straightforward. In nutrition, it pops up in tablets and capsules, providing doctors and patients a simple, consistent way to address iron deficiency. The product comes in different mesh sizes and with varying levels of trace metals, depending on what industry it's destined for.
Physical & Chemical Properties
Ferrous sulfate monohydrate lands on the scale with a molecular weight of about 169.93 g/mol. It appears pale blue-green, and it will turn yellowish on exposure to air—the sign that oxidation to ferric sulfate is taking place. Standard storage calls for a cool, dry place because the compound easily absorbs moisture from the air. As a reducing agent, ferrous sulfate can initiate a handful of classic chemical reactions, which makes it handy for water treatment, pigment production, and laboratory synthesis. Mix it in water, and it dissolves with ease, though it settles out quickly if left alone, which farmers know from mixing tank experience. The monohydrate version, compared to heptahydrate, carries less water and packs more iron per kilogram.
Technical Specifications & Labeling
Products must meet clear targets for iron content—typically 30-33% by mass—and moisture content. Most regulations, like those from the USP or EP, spell out allowable levels of heavy metals, arsenic, and insoluble residue. Labels reflect this, showing minimum iron guaranteed, moisture range, and sometimes pH value of a standard water solution. Lot number, manufacturing date, and hazard symbols also appear; a quick glance at any bag tells you if it aligns with legal and industry benchmarks. My time in compliance support for bulk shipments taught me that paperwork can make or break a deal—missing a certificate of analysis throws everything off. Reputable suppliers show lab certificates, which adds a layer of confidence for anyone using the product in food, medicine, or animal feed.
Preparation Method
The typical route to ferrous sulfate monohydrate starts with iron and sulfuric acid. Scrap iron reacts with diluted acid in controlled reactors, driving off hydrogen gas and leaving a solution of ferrous sulfate. Filtration removes insolubles, and cooling the solution leads to crystal formation and water separation. The crystals then go through drying to reach the single-molecule water content that defines monohydrate. Modern facilities recover process heat, capture acidic vapors, and recycle excess water, which drops emissions and water use per ton. Some sources come from by-products of steel pickling plants; here, the route swaps primary iron for recycled material but lands at similar purity if managed well. The waste streams carry iron-rich sludge and acid residues, both tightly regulated to avoid environmental damage.
Chemical Reactions & Modifications
Ferrous sulfate takes part in simple and complex reactions. In water treatment, it acts as a coagulant, reacting with hydroxides to form flocs that sweep up small particles. Expose it to oxidizers, like chlorine or air, and it forms ferric sulfate or iron oxides—rust, essentially. In pigment work, chemists combine it with tannins from plants or with potassium ferrocyanide to make inks and Prussian blue pigment. You can convert it to other iron salts with acidic or basic treatment, broadening the reach in industries from dye to fertilizer. Sometimes, companies coat ferrous sulfate granules to slow release or improve flow, especially for use in granular NPK blends for farming.
Synonyms & Product Names
Ferrous sulfate monohydrate carries a range of labels in trade and research: iron(II) sulfate monohydrate, green vitriol, copperas, and in some pharmacopoeias, it’s referred to as iron sulfate, mono. Each name marks out a slightly different context, but in practice, they all zero in on the same compound. In animal feed, it regularly appears as “feed-grade iron.” In water treatment circles, folks usually just call it “ferrous” or “FS monohydrate.” On the packaging shelf, you see brand names and generic designations, all referencing the content of Fe and the water of crystallization.
Safety & Operational Standards
Handling this compound calls for caution and respect for health. Inhalation of dust poses risks; direct contact sometimes irritates skin or eyes. Most factories require gloves, goggles, and dust masks for workers, and many sites enforce closed systems or dust extraction setups. In the event of accidental ingestion, especially among children, iron salts rank among household hazards—cases of overdose are not rare and can be very severe, sometimes fatal. Emergency responders follow strict medical protocols for treatment. Regulatory agencies such as OSHA and the European Chemicals Agency require detailed safety data sheets, labeling for hazard communication, and records of employee training. Spillage must never flow into water bodies because large discharges harm fish and aquatic life—iron clouds the water, settles muck, and alters oxygen levels.
Application Area
Ferrous sulfate monohydrate spreads its reach from agriculture to industry to medicine. Farmers use it to fix iron-poor soils, especially for high-demand crops like citrus and blueberries. It’s also blended into animal feeds to address iron deficiency anemia, a particular concern for piglets and some poultry species. Water utilities reach for ferrous sulfate monohydrate to treat municipal supplies, both for coagulation and to reduce emerging contaminants through advanced oxidation. In pharmaceuticals, it forms a core part of iron supplements—chewable tablets, syrups, and capsules. A run through the ingredient list on popular multivitamins confirms this. Its use in pigment and dye manufacture, while old, persists for niche products and for specialty printing inks. Many cement and concrete producers use it to control chromate levels in finished products, an important worker safety measure in Europe.
Research & Development
Scientists continue to experiment with new coatings and formulations aimed at improving uptake in both crops and people. Nanoparticle suspensions, slow-release formats, and chelate blends pop up in journals and behind company doors. Research teams look for ways to blend ferrous sulfate with organic acids or natural carriers, with the aim of boosting absorption and slashing gastrointestinal upset in humans. In agriculture, field trials focus on minimizing leaching and runoff while sustaining or even increasing crop response. New methods for removing trace contaminants, such as lead or cadmium, during manufacture, represent a quiet but important front, driven by tightening rules and market demand for ultra-low impurity products. Each improvement comes down to reducing side effects, improving stability, or refining the delivery system—goals that all matter to both end users and regulators.
Toxicity Research
Toxicology data on ferrous sulfate show why handling standards matter. Acute ingestion of as little as a gram or two can trigger vomiting, abdominal pain, and, when the dose gets high enough, organ dysfunction or death, especially in children. Chronic exposure, usually through dust in workplaces, risks iron overload, which can damage the liver or other organs over time. Several studies point out that, in animals, excessive dietary levels stoke oxidative stress. Clinical research shows most people tolerate recommended doses well, but every year, poison control centers still list iron salts near the top for childhood accidental poisoning. Guidelines push for child-proof packaging on supplements and clear warning marks on industrial products. Researchers continue, too, to track long-term impacts of low-level environmental exposure, noting how it can hit aquatic ecosystems even when human health is not apparently at risk. This has pushed industry to improve wastewater treatment and product stewardship.
Future Prospects
As global food production pushes for higher yields from limited soil, demand for iron-containing fertilizers stands to climb. Ferrous sulfate monohydrate, by virtue of price and availability, will keep a good share of this space. In the medical field, growing populations and changes in dietary preference mean more people need iron support, especially children and women of reproductive age. Gains in refining and formulation technology look set to make products safer and easier to handle—less dust, fewer contaminants, more options for end users. Environmental regulations keep getting stricter, forcing suppliers to clean up production, close pollution loops, and offer better documentation. Some start-ups focus on extracting ferrous sulfate from industrial waste, closing loops that both reduce landfill and slash raw material usage. Innovation in delivery—microcapsules, liquid suspensions, and mixes with probiotics—will likely make iron supplementation more effective and, hopefully, easier on the stomach. More research on bioavailability, side effect reduction, and environmental impact will keep pushing the industry ahead, balancing performance with safety and sustainability.
What is Ferrous Sulfate Monohydrate used for?
How Ferrous Sulfate Monohydrate Steps In
Ferrous sulfate monohydrate rarely shows up in big headlines, but many people depend on it every day. Doctors have turned to this mineral compound for decades. It helps treat iron deficiency anemia, a condition that leaves people feeling run-down, weak, and foggy-headed. As someone who has supported family members with iron deficiency, I’ve seen the impact of missed doses. Energy levels stall, and daily routines feel harder. Ferrous sulfate monohydrate works by providing a boost of iron, giving red blood cells what they need to carry oxygen through the body. With regular use and careful medical guidance, patients often get their strength back within weeks.
Feed and Food Additives: Supporting Nutrition
Beyond medicine cabinets, ferrous sulfate monohydrate finds plenty of work outside the doctor’s office. Farmers rely on this compound as a basic animal feed additive. Pigs and poultry, in particular, need enough iron for healthy growth. Without it, young animals fail to thrive. The World Health Organization and other serious authorities flag iron deficiency as a problem both in humans and livestock. So, adding ferrous sulfate to feed formulas helps deliver those missing nutrients.
You sometimes see it in fertilizers, too. Lawns, gardens, and even sprawling golf courses show greener colors thanks to added iron. Where I live in the Midwest, yellow patches in the yard hint at iron-starved soil. Spraying or spreading a small amount of ferrous sulfate monohydrate quickly reverses those pale streaks. Crops, too, find benefit. Grain farmers know that iron matters for plant health, boosting yields and keeping plants free from disease.
Industrial Uses: Taming Water and Preventing Pollution
City water engineers reach for ferrous sulfate monohydrate during water treatment. Iron compounds bind with phosphates, which are a troublemaker in municipal wastewater plants. Too many phosphates feed algae blooms, choking lakes and rivers and putting fish at risk. As part of a larger treatment plan, ferrous sulfate monohydrate reduces these pollutants before they flow downstream.
Other industries use it as a coagulant. Paper mills, textile dye houses, and mining operations take advantage of its ability to settle small particles and clean up effluents. My neighbor works at a paper plant and describes regular shipments of ferrous sulfate as part of the “hidden backbone” of clean production.
Safety and Responsible Use
Ferrous sulfate monohydrate deserves respect. Swallowing too much can harm children; proper labeling and locked cabinets matter at home. Industrial scale applications follow strict handling rules to keep workers safe. It’s also important to guard against soil and water buildup, which can lead to environmental imbalances. Many environmental agencies track its use and set safe thresholds based on years of data.
As iron deficiency keeps filling headlines in health and agriculture, ferrous sulfate monohydrate remains part of the toolkit. It blends science, tradition, and practical know-how into something you rarely notice, but would quickly miss if it disappeared.
What is the recommended dosage of Ferrous Sulfate Monohydrate?
Iron deficiency leaves people tired, listless, and slow to shake off infections. That’s why many reach for iron supplements like ferrous sulfate monohydrate. Dosage matters. Getting it right makes the difference between boosting your iron stores and feeling queasy, constipated, or worse. The U.S. National Institutes of Health points to an adult dose of about 65 mg of elemental iron (which comes from around 200 mg of ferrous sulfate monohydrate once or twice daily, depending on the tablet’s size and what your doctor says). Children’s doses depend on body weight and the reason for the supplement—so no adult guesses for kids, ever.
Experience and Caution: Why Following Guidance Matters
After seeing plenty of people struggle with stomach cramps, constipation, or black stools after taking more than recommended, it’s clear: respecting the standard dose isn’t fussing—it’s necessary. Iron pills hit the gut hard. A bit too much piles on nausea for some. Cutting corners—taking irregular doses or combining with antacids—prevents your body from absorbing what it really needs. Skipping advice from your doctor or pharmacist doesn’t speed up recovery. It might turn matters worse, such as accidental iron poisoning, especially in children. The Centers for Disease Control and Prevention often reminds about accidental overdose being a leading cause of fatal poisoning in children younger than six.
How to Get the Most From Your Iron Supplement
Most doctors advise swallowing iron with water or juice on an empty stomach. That ups absorption. Eating foods rich in vitamin C—like an orange or some bell pepper—alongside can help your body grab every bit of that supplement. Calcium from milk or supplements, tea, and coffee can block your efforts. Iron can stain teeth or taste metallic, but rinsing your mouth or using a straw can dodge this problem. Track symptoms. If side effects crop up, like sharp cramps or trouble swallowing, talk to your doctor straight away. They might lower the dose or switch brands. Slow-release tablets or a lower starting dose can make a world of difference for some folks.
Who Shouldn’t Use Iron Supplements Without Medical Direction?
People with conditions like hemochromatosis or frequent blood transfusions store too much iron already. Another risk comes with certain stomach issues, or those dealing with chronic kidney disease. The body can only process so much iron. Piling on more doesn’t solve the core problem—it lands you in the emergency room. Always consult a healthcare provider before starting a new supplement, especially for children, pregnant women, or people already taking other medications. Pregnant women sometimes need more iron, but the dose still depends on blood tests and medical advice, not guesswork.
Practical Steps Toward Safer Supplementation
Sticking to a routine helps. Set reminders on your phone or pair your pill with the same meal each day. Store iron tablets well out of reach of kids—locked up, not just stuck in a high cupboard. If you miss a dose, don’t double up later. Just carry on as normal. Monitor how you feel each week. Share those details at your next doctor appointment. That conversation lets your healthcare provider fine-tune the dosage, check your blood levels, and spot anything out of the ordinary before it grows larger.
In the end, one-size-fits-all doesn’t work for health. Individual needs differ based on age, diet, and health history. Science continues to emphasize personalized healthcare and careful supplementation. A steady hand, clear goals, and regular conversations with health professionals keep things safe and effective for all.
Are there any side effects of taking Ferrous Sulfate Monohydrate?
Iron Pills Aren’t Magic Bullets
Ferrous sulfate monohydrate has a place in my own medicine cabinet, thanks to years of low energy and test results pointing to iron deficiency. Out of all the supplements and pills people take, iron is one of the most common. Doctors hand it out for anemia, pregnancy, low iron in athletes, and all kinds of other situations where blood tests show someone’s iron stores have dropped. The idea makes sense — you need iron to make hemoglobin, which hauls oxygen everywhere your body needs it. If your cells don’t get enough of that, energy tanks. Breathing feels heavy, stairs become a real hurdle.
Most people reach for ferrous sulfate monohydrate in the grocery store aisle or fill a prescription, hopefully after talking with a doctor who checked their bloodwork. For all its benefits, iron pills do come with some real drawbacks, and I’ve seen them firsthand.
The Gut Takes the Biggest Hit
Digestion notices these pills before anything else does. After the first few doses, I battled stomach cramps that wouldn’t let me sleep. Constipation quickly followed, along with heartburn. Studies back up what my gut learned: about half the people who take iron pills get some kind of stomach trouble, especially at higher doses. Gas, dark stools that look worrisome until you learn that it’s normal, queasiness after food — all of this can be expected. Not everyone gets the same severity. One friend barely notices. Another can’t make it through a day without feeling queasy.
Doctors sometimes switch the iron form, lower the dose, or suggest taking it with a bit of food to dial things down. Vitamin C can help the gut soak up more iron, but too much orange juice or ascorbic acid can irritate a sensitive stomach.
Less Obvious Reactions
Beyond the bowels, iron can cause other issues. Some users complain of a metallic taste. Allergic reactions—itching or swelling—rarely show up but shouldn’t be ignored. Taking too much iron, especially if kids get into the bottle, can turn dangerous fast. Poison centers receive calls about accidental overdoses every week. High doses over time may stress the liver, especially in people with conditions like hemochromatosis, where the body stores way too much iron without letting go. This doesn’t happen overnight, but it’s worth a mention because testing for iron overload is cheap and saves a world of trouble later.
Prevent Trouble Before It Starts
Doctors and pharmacists have their work cut out for them in picking the right dose and form of iron. Time-release tablets, lower doses split through the day, or sometimes switching to a liquid can cut down the gut pain. Patience helps. Starting slow, working up to a full dose, or even taking a supplement every other day—these approaches keep many people on iron long enough to see real improvement without quitting early from the side effects.
Food sources like beans, meat, leafy greens, and fortified cereals can bridge the gap for people who find pills too hard on their system, but they often aren’t enough when blood levels are seriously low. Still, anyone struggling on iron pills should look beyond medicine for extra help—changing up the diet goes a long way.
Ask Questions and Pay Attention
I learned the most by keeping an eye out for changes and reporting back to my doctor early. Catching problems beats worrying in silence. Regular blood checks track improvement and make sure things stay on the right path. Suffering in silence with cramps, constipation, or nausea doesn’t impress anyone. Iron pills do their job, but they don’t suit everyone the same way. Honest conversation helps find the right balance.
Ferrous sulfate does what it promises, but respecting its limits and knowing the risks makes the path much smoother for people looking to restore their health, energy, and quality of life.
How should Ferrous Sulfate Monohydrate be stored?
Understanding What’s at Stake
Ferrous sulfate monohydrate shows up in agriculture, nutrition, and water treatment. Storing it right doesn’t just tick off a safety checklist; it keeps products useful and people safe. Over the years, I’ve seen plenty of supply rooms and warehouses where corners get cut, and it’s usually not out of laziness. For many, iron supplements and similar materials seem harmless. That sort of thinking often feeds problems down the line.
Why Moisture Makes Trouble
Ferrous sulfate monohydrate draws in water from the air. I remember a shipment that once arrived clumped together like wet flour because someone left the pallet beside an open loading dock. Clumping isn’t just messy. Exposure to moisture starts chemical changes, dropping effectiveness and even turning the compound into something else. Humidity spikes in a storage room can cause discoloration and hardening, which means trouble for mixing, dosing, or applying it.
The Risks of Unprotected Storage
Corrosion delivers another headache. Steel bins or shelving without coatings attract rust quickly. I’ve witnessed rusty trails where carelessly stored bags sat on metal grates. Rust contaminates products and machinery. When iron content matters, you can’t afford even small changes. Storing direct on concrete also brings problems: moisture wicks up under bags or drums, feeding chemical changes right through the packaging.
Practical Strategies from the Field
Smart storage starts with a dry, shaded room. Facilities often use dehumidifiers to control air moisture. Good packaging isn’t just about plastic wrapping; inner liners and sealed bags matter. Based on my own warehouse work, double-bagged shipments rarely run into trouble. I’ve also seen manufacturers ship pallets shrink-wrapped with moisture barriers, especially through humid seasons.
Keep materials off the floor using pallets, and avoid any direct contact with brick or concrete. In places with dramatic temperature swings, insulation makes a real difference. Monitoring humidity with a sensor isn’t science fiction or overkill—it’s practical and prevents loss. Labeling helps everyone stay sharp: clear labels cut mistakes, especially for crews who handle dozens of chemicals in one room.
Health and Environmental Safety
Personnel carry the risk, too. Dust from ferrous sulfate monohydrate irritates the lungs and eyes. If spills happen, a contained and dry storage area means less chance of accidents spreading. Wearing gloves and masks isn’t just a box-ticker for compliance. Staff working day in and day out appreciate straightforward protective measures. Every year, people end up in clinics after unexpected exposure to what looked like a harmless powder.
Aim for a Culture of Care
Following storage practices isn’t about paranoia or red tape. It’s about respect for the materials and the people working with them. Ferrous sulfate monohydrate sits on countless shelves worldwide, often out of sight. Whether it’s industrial, agricultural, or small-batch use, giving attention to storage details prevents waste, keeps supply chains consistent, and supports public health. Simple steps make the difference between reliable supply and ongoing headaches—any manager or technician will tell you the same.
Is Ferrous Sulfate Monohydrate safe during pregnancy?
Iron Deficiency Isn’t Rare in Pregnancy
During pregnancy, women’s bodies work overtime. The body needs iron to build new blood cells and get enough oxygen to the developing baby. The challenge comes when food alone doesn’t always cut it. Many pregnant women run low on iron, which leads to fatigue, pale skin, or even trouble focusing. Health professionals commonly spot low iron on blood tests and then suggest supplements. Ferrous sulfate monohydrate, a type of iron supplement, often pops up in these conversations.
Is Ferrous Sulfate Monohydrate Safe?
Doctors have used ferrous sulfate monohydrate for decades. The U.S. Food and Drug Administration lists iron salts, including ferrous sulfate, as safe during pregnancy if taken as directed. Obstetricians confirm its place as a go-to iron supplement when bloodwork shows iron-deficiency anemia.
Iron pills, including ferrous sulfate monohydrate, come with common side effects. Upset stomach, constipation, or a metallic taste aren’t unusual. Doctors may suggest taking supplements with food to limit stomach troubles, though this can lower iron absorption a bit. Drinking plenty of water and eating more plants high in fiber helps manage constipation.
Getting the correct dose makes a difference. Too much iron can build up in the body, causing harm to mother and baby. Blood tests before and during pregnancy guide dosing. Pregnant women should only take iron under supervision, not out of guesswork or advice from friends.
The Science Behind Iron’s Role
Research highlights how crucial iron is during pregnancy. The Centers for Disease Control and Prevention say anemia in pregnancy raises the risk of early birth and low birth weight. The World Health Organization estimates that around 40% of pregnant women worldwide live with anemia. Iron supplement programs, often using ferrous sulfate monohydrate, tackle these risks head-on.
Ferrous sulfate monohydrate wins praise because it delivers a reliable amount of iron and keeps costs low. Health agencies in the U.S., U.K., and Australia include it in their official guidelines for pregnancy care. Evidence supports its ability to raise iron stores and boost energy, oxygen delivery, and even mental sharpness in tired moms.
Practical Ways to Stay Safe
Pregnant women can take steps to ensure iron supplements do their job. Regular prenatal appointments include bloodwork and allow the doctor to check how much iron is needed. It pays to take iron as recommended by a health professional. Swapping to a different iron product or lowering the dose can happen if side effects bother someone. Doctors may suggest pairing iron with vitamin C-rich foods, such as oranges, to help the body absorb more.
My own experience watching family members go through pregnancy taught me how frustrating iron deficiency can feel. Tiredness doesn’t just wear a person down physically; it affects mood and even relationships. The right supplement makes an obvious difference in weeks.
Thoughts on Moving Forward
No one supplement replaces a balanced diet and good prenatal care. Ferrous sulfate monohydrate offers an effective way for pregnant women to correct iron deficiency, guided by clear science and evidence from decades of practice. Any pregnant person thinking about supplements should start with an honest conversation with a doctor. Being open about side effects and staying up to date with blood tests lets both mom and doctor work together for the best outcome.
| Names | |
| Preferred IUPAC name | iron(II) sulfate monohydrate |
| Other names |
Iron(II) sulfate monohydrate Green vitriol Copperas monohydrate Ferrous sulphate monohydrate Iron sulfate monohydrate |
| Pronunciation | /ˈfer.əs ˈsʌl.feɪt ˌmɒn.oʊˈhaɪ.dreɪt/ |
| Preferred IUPAC name | iron(2+) sulfate monohydrate |
| Other names |
Iron(II) sulfate monohydrate Green vitriol monohydrate Copperas monohydrate Ferrous sulphate monohydrate |
| Pronunciation | /ˈfɛr.əs ˈsʌl.feɪt ˌmɒn.oʊˈhaɪ.dreɪt/ |
| Identifiers | |
| CAS Number | 17375-41-6 |
| Beilstein Reference | 1699071 |
| ChEBI | CHEBI:75832 |
| ChEMBL | CHEMBL1201608 |
| ChemSpider | 22878 |
| DrugBank | DB06744 |
| ECHA InfoCard | 13e3bfaf-6a6f-4d61-bcb7-7d3767e4e7e4 |
| EC Number | 231-753-5 |
| Gmelin Reference | 563242 |
| KEGG | C00444 |
| MeSH | D003680 |
| PubChem CID | 251983 |
| RTECS number | BQ9300000 |
| UNII | VVI60WSQJK |
| UN number | UN3077 |
| CAS Number | 13463-43-9 |
| Beilstein Reference | 1369650 |
| ChEBI | CHEBI:75832 |
| ChEMBL | CHEMBL1201561 |
| ChemSpider | 86306 |
| DrugBank | DB03147 |
| ECHA InfoCard | 03cfdd8f-3e53-46c9-b63b-317f9779e98b |
| EC Number | 231-753-5 |
| Gmelin Reference | 82263 |
| KEGG | C06432 |
| MeSH | D017384 |
| PubChem CID | 25138 |
| RTECS number | NO4565500 |
| UNII | C1J0T5G947 |
| UN number | UN3077 |
| Properties | |
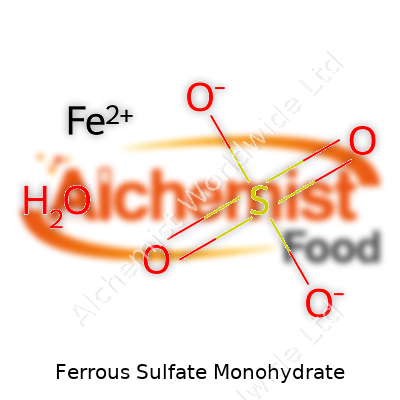
| Chemical formula | FeSO4·H2O |
| Molar mass | 169.93 g/mol |
| Appearance | Light greenish crystalline powder |
| Odor | Odorless |
| Density | 2.0 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | ~1.99 |
| Basicity (pKb) | 7.2 |
| Magnetic susceptibility (χ) | '−46.2 × 10⁻⁶ cm³/mol' |
| Dipole moment | 9.02 D |
| Chemical formula | FeSO4•H2O |
| Molar mass | 169.93 g/mol |
| Appearance | A pale blue to blue-green crystalline powder |
| Odor | Odorless |
| Density | 2.0–3.0 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.5 |
| Basicity (pKb) | 6.2 |
| Magnetic susceptibility (χ) | '−46.4 × 10⁻⁶ cm³/mol' |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 151.0 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | −930.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1598 kJ/mol |
| Std molar entropy (S⦵298) | 151.0 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -928.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1560 kJ/mol |
| Pharmacology | |
| ATC code | B03AA07 |
| ATC code | B03AA07 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-1 |
| Lethal dose or concentration | LD50 Oral Rat: 1,520 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, Rat: 1518 mg/kg |
| NIOSH | N0397 |
| PEL (Permissible) | PEL: 15 mg/m3 (total dust), 5 mg/m3 (respirable fraction) |
| REL (Recommended) | 27 mg |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Lethal dose or concentration | LD₅₀ (oral, rat): 1,519 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 1,384 mg/kg |
| NIOSH | WI009 |
| PEL (Permissible) | PEL: 15 mg/m³ (total dust) |
| REL (Recommended) | Recommended Exposure Limit (REL) for Ferrous Sulfate Monohydrate is "1 mg/m³ (as iron, time-weighted average)". |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Iron(III) sulfate Iron(II) chloride Iron(III) chloride Ferrous fumarate Ferrous gluconate |
| Related compounds |
Ferrous sulfate heptahydrate Ferric sulfate Ferrous fumarate Ferrous gluconate Iron(II) chloride Iron(III) chloride |

