Ferrous Glycinate: A Down-to-Earth Review of an Essential Iron Supplement
Historical Development
Decades ago, iron supplements mostly showed up in plain tablets or in mineral mixes that had a distinct metallic taste and a reputation for causing upset stomachs. Over time, science pushed new chelated forms of iron into the market, searching for gentler, better-absorbed alternatives. Researchers experimenting with amino acid chelation landed on ferrous glycinate as a way to bind iron to glycine, allowing for smooth delivery inside the gut. This blend grew traction among nutrition scientists and food technologists aiming to beat the barriers of older ferrous salts. The supplement started showing up more in clinical trials, wining supporters for its higher bioavailability and milder gastrointestinal effects. Today, it's become a staple in food fortification strategies in countries battling iron deficiency, and manufacturers see it as a reliable mineral additive in everything from cereals to infant formula.
Product Overview
Ferrous glycinate stands as a chelated iron source, where iron teams up with glycine, an amino acid. By pairing up with glycine, iron sidesteps the common drawbacks of ferrous sulfate and similar raw mineral salts—mainly the poor absorption and those all-too-common digestive complaints. Nutrition companies market ferrous glycinate in several forms, including bulk powders, capsules, granules, and sometimes as part of pre-mixed nutrient premixes. Adult and pediatric formulas take advantage of its taste neutrality, which helps cut the metallic tang often reported with iron supplements. The form and grade chosen often depend on the final food or beverage use, as well as the regulations in the target country.
Physical & Chemical Properties
This compound usually looks like a light tan or off-white powder, flowing freely and dissolving well in water. A key property: it shows strong stability under storage and food processing conditions, which means it holds up under heat and humidity far better than some competitors. Its solubility rate supports use in both dry mixes and liquid solutions, and the chelation reduces unwanted chemical interaction with other nutrients in fortified foods. Each molecule has iron linked in a bidentate coordination with glycine, locking the mineral in an organic wrapper and shielding it from quick oxidation or precipitation in food matrices. Because of glycine’s small size, the chelate slips through the intestine’s absorption pathway without competing as much with other minerals.
Technical Specifications & Labeling
Manufacturers usually supply ferrous glycinate to specific standards, pointing to minimum iron content, total trace metals, moisture limits, and microbial purity. Labels used in food and supplement products rely on local rules—often naming it “ferrous bisglycinate” or “iron bisglycinate.” In the US, the FDA classifies it as a “Generally Recognized as Safe” (GRAS) ingredient. European guidelines treat it similarly. Technical datasheets provide details on mesh size, solubility, pH, and assay methods. The iron content by mass typically ranges from 17% to 20%, depending on the hydration state, so manufacturers adjust blend levels to match nutritional targets, keeping within safety limits outlined by nutrition authorities.
Preparation Method
Most commercial synthesis of ferrous glycinate starts with ferrous sulfate, which reacts with glycine under controlled pH, low oxygen, and room-to-warm temperature. The process requires careful addition of glycine and continual mixing, usually under a nitrogen atmosphere to guard against oxidation. Filtration follows, and the product gets dried under vacuum or with low-heat air. Some manufacturers add a step to granulate or microencapsulate the powder, which boosts its flow properties and resistance to clumping. Quality control labs test for iron oxidation states and residual chemicals. The preparation approach aims for high purity, low contamination, and tight particle size distribution so that food or supplement makers can dose the product accurately.
Chemical Reactions & Modifications
In storage and inside food products, ferrous glycinate resists many of the reactions that plague ferrous sulfate. The chelate bond remains stable under common pH ranges found in the human gut, only breaking open once it reaches specific digestive enzymes in the intestine. In acidic or oxidizing environments, such as during food processing or inside stomach acid, the iron stays more protected than in naked salts. Some research groups have tested co-chelation, where glycine partners with vitamin C or other amino acids to further increase stability or solubility. Chemical modification, like micronization or encapsulation, mainly focuses on enhancing shelf life and mixing ability with tough food matrices, especially those high in fat or fiber.
Synonyms & Product Names
People in the nutrition world know ferrous glycinate by a few names—“iron bisglycinate,” “ferroglycine,” “bisglycinate iron,” and “ferroglycinat” in some regulatory registers. Ingredient suppliers sometimes brand it under proprietary trademarks, especially if they tweak the chelation procedure or particle technology. On supplement bottles, you might spot it as “gentle iron,” or see it lumped with broader descriptors like “amino acid chelated iron” or “chelated iron bisglycinate.” No matter the branding, it’s the strong glycine bond that sets the compound apart, not just the iron content.
Safety & Operational Standards
Dietary and food-grade ferrous glycinate has won approval from major global safety agencies, based on a strong record of clinical data and toxicological reports. The European Food Safety Authority (EFSA) and the US FDA both recognize it as safe for general use at approved iron levels. Production plants follow GMP, HACCP, and ISO 22000 standards to ensure traceability, hygiene, and microbial control. Facilities watch for heavy metal contaminants, allergen risk, and consistent iron yields by running batch tests. Operational teams wear protective gear and stick to strict dust control practices, as iron supplements can be irritants if inhaled or handled in bulk without safeguards.
Application Area
Ferrous glycinate’s popularity covers a wide swath of the market: supplement pills and capsules for adults and children, baby foods, nutritional powders, beverages, and vitamin blends. Hospitals and clinics use it for patients with iron deficiency anemia who have trouble tolerating traditional iron salts. International nutrition programs and NGOs often pick this compound for food fortification projects in regions hard hit by iron deficiency, such as fortified rice, wheat flour, or dairy substitutes. Food scientists appreciate the taste stability, since it doesn’t turn milk or cereal metallic or off-flavored, making compliance much higher in real-world settings. Beverage and sports-nutrition brands go for this form because its solubility supports liquid fortification without sediment problems.
Research & Development
Academic labs and supplement brands invest resources chasing ways to improve absorption and reduce side effects. Modern studies dig into how ferrous glycinate gets taken up compared to older salts, using radiolabeling and absorption markers. Major trials measure hemoglobin response, serum ferritin, gastrointestinal complaints, and long-term iron repletion in both adults and kids. Some groups run head-to-head comparisons in real-life food matrices, such as fortifying bread, milk, or ready-to-eats, then tracking taste, stability, and delivered iron. Every few years, the science community publishes meta-analyses or systematic reviews weighing evidence from dozens of trials, helping regulatory agencies set policy. Industry partnerships fund work to optimize the manufacturing process, improve international supply chains, and design new formulations that blend this chelate with other micronutrients like zinc, folic acid, or vitamin B12.
Toxicity Research
Toxicology studies agree on a wide safety margin for ferrous glycinate at recommended levels, both in animal models and human case studies. Tests check short- and long-term exposure, looking for kidney, liver, or gut reactions. Iron overload risk stays tied mostly to underlying genetic conditions, like hemochromatosis or rare iron metabolism disorders, rather than routine supplement use. No evidence links ferrous glycinate to carcinogenic, mutagenic, or reproductive harm. Some research groups keep pushing for even tighter upper limit data for infants or at-risk populations, but data currently point to a safety profile matching or exceeding other approved iron compounds. Rare allergic reactions or side effects tend to center more on excipients or product quality than the chelated iron itself.
Future Prospects
Chronic iron deficiency still tops the charts for global micronutrient challenges, and food technology keeps searching for better ways to meet this gap. Ferrous glycinate holds strong promise for meeting the needs of vulnerable groups—infants, pregnant women, and those living in poverty—without causing the compliance and side-effect problems that set back older iron products. Manufacturers look for green chemistry advances to cut costs and carbon footprint, as well as customized blends tuned for local diets. As governments and organizations expand food fortification laws, demand for highly stable, absorbable iron forms will keep climbing. Researchers keep studying genetic interactions, microbiome effects, and optimal formula blends, aiming to help more people safely hit daily iron targets worldwide.
What is Ferrous Glycinate used for?
The Role of Iron in Daily Health
I’ve watched family, friends, and even myself grow up hearing about iron. Not the kind that turns rusty in the garage—real iron that runs in the blood. It brings oxygen around the body, helps in making energy, and keeps the mind clear. Some people, especially women and kids, struggle to get enough. You might see the signs as tired faces, dull skin, or classmates who just can’t seem to focus in school.
What Makes Ferrous Glycinate Different?
I remember taking some of those cheap iron tablets in college. After a while, the stomach cramps and that awful metallic taste made me quit quicker than any diet. Doctors later showed me alternatives. Ferrous glycinate came up as a better choice. This compound brings iron together with glycine, an amino acid, so the nutrient gets into the body more smoothly. It’s less likely to upset the digestive system, and it doesn't change the taste of foods or drinks either.
Who Needs It Most?
Young women, pregnant people, kids, and older adults tend to get hit hardest by iron deficiencies. Heavy monthly cycles or pregnancy can drain iron stores. Kids grow fast and burn through iron just to keep up. Grandparents, too, sometimes stop enjoying red meat or leafy vegetables. Diet alone falls short. Ferrous glycinate makes sense here. It gets absorbed very well, even with food around—something other iron supplements struggle with.
Data That Counts
Clinical studies back this up. The World Health Organization notes that iron deficiency is the most common nutrient shortage globally. Over 1.6 billion people face it. Trials show that ferrous glycinate can raise iron levels without the side effects that come with older salts like ferrous sulfate. Patients taking it stick with the treatment longer, and blood results show real improvement. Constipation, black stools, and stomach pain drop off. It’s a win for real-world results, especially in children who fight iron drops and gummies.
Real Life Solutions
Parents who struggle to get their children to swallow pills or eat iron-rich foods find some relief in ferrous glycinate syrups or powders. Food companies now blend it into cereals and infant formulas in doses that boost iron but do not mess with taste or color. Health workers in low-income regions use these products in nutrition programs. Even athletes look to this supplement as they replace lost iron after long training.
Looking After Your Own Health
I always encourage checking with a doctor before grabbing any iron supplement. Get a blood test. Not everyone needs more iron, and too much causes problems like organ strain or stomach pain. For people who truly need an extra boost, ferrous glycinate stands out. It delivers more iron, easier on the stomach. No one likes choking down chalky tablets or skipping doses just to avoid cramps. This option gives you iron without the fight.
Is Ferrous Glycinate better absorbed than other iron supplements?
Real Struggles With Iron Deficiency
Iron deficiency brings more than tiredness. People dealing with low iron notice hair shedding, pale skin, and shortness of breath taking over daily life. After years of hearing friends share frustrations with tablets that upset their stomach or just don’t seem to give energy back, I started to dig into how different forms of iron get used by the body.
The Absorption Game
Doctors usually start people on simple iron tablets like ferrous sulfate. It’s cheap and easy to find, but it leaves folks doubling over with nausea or running to the bathroom. The gut doesn’t always handle it well, and leftovers move through the system instead of helping build up iron stores.
Ferrous glycinate gets praise from many who’ve tried other types without luck. The idea: binding iron to the amino acid glycine helps it sneak across the gut wall more easily, where the body grabs it before it causes irritation. Several scientific reviews back up better absorption for ferrous glycinate over standard salts. For example, a 2019 study in the American Journal of Clinical Nutrition showed that women with iron deficiency anemia saw larger increases in ferritin from ferrous bisglycinate (which is similar in action to ferrous glycinate) compared to other forms. The protein binding seems to matter—results suggest glycinate forms skip the harsh stomach reactions, leading to higher compliance and better long-term results.
Gut Feelings: Why It Matters in Real Life
Side effects drive many people off iron therapy. Ferrous sulfate and gluconate cause cramps, constipation, or a metallic taste. I’ve watched patients give up after only a week. If ferrous glycinate can provide nearly double the absorption at a lower dose, as shown in comparative trials, that changes everything for those sensitive stomachs. More iron in the bloodstream per capsule means folks heal faster, and the motivation to stick with treatment grows.
People with chronic conditions—celiac disease, heavy menstrual cycles, or vegan diets—often battle persistent low iron. Some athletes never catch up using standard supplements, fighting exhaustion day after day. Ferrous glycinate offers a gentler alternative, which research and direct experience both support.
What the Science Backs Up
Meta-analyses offer some clear answers. Ferrous glycinate typically reaches 2-4 times greater bioavailability than ferrous sulfate at equal doses. That translates to higher hemoglobin and ferritin numbers across months. The World Health Organization acknowledges chelated forms like ferrous glycinate as safe, especially for tough cases such as pregnancy or chronic anemia.
What Works for You
No supplement works for absolutely everyone. Some people react to glycine. Some tolerate plain old ferrous sulfate without complaint. Still, the science and stories stack up: ferrous glycinate often relieves the very symptoms that keep people from sticking with their treatment plan. Health professionals look at lab changes—iron, hemoglobin, ferritin—not just the label on the bottle. An approach that helps keep stomachs calm while bringing iron levels back means people show up for work, school, and family life again.
Pharmacies now offer more options—chelated and regular forms sit side by side. Finding the best iron supplement isn’t about trends but about what actually helps someone feel strong and healthy again. Anyone who feels wiped out all the time, despite taking their pills, can ask their doctor or dietitian for a different iron source. Ferrous glycinate deserves a spot among the top recommendations for those struggling to bring their iron back in balance.
What are the side effects of Ferrous Glycinate?
Understanding Ferrous Glycinate
Ferrous glycinate shows up on the label when someone needs an iron supplement that treats iron deficiency. Doctors write out these prescriptions all the time for people with anemia or anyone not getting enough iron from food. Ferrous glycinate releases iron in a way that’s easier on the gut than many older iron salts like ferrous sulfate. It sounds almost gentle, but that doesn’t mean it leaves everyone off the hook when it comes to side effects.
Digestive Troubles
Gut reactions show up first on the list of complaints. The classic iron pill comes with threats: stomach cramps, nausea, and runs to the bathroom. Ferrous glycinate wins some points by being less harsh, but stories from real people echo through clinics. Constipation hits hard for some, bringing bloating or strain. Others deal with diarrhea or stomach aches that make daily routines uncomfortable. In my own circle, a friend juggling iron pills found herself skipping doses because her stomach paid the price each morning. She tried taking it with food but noticed the stubborn fatigue coming back—food trims the side effects, but it can block how much iron you actually absorb. From a science angle, this balancing act matters: researchers found that taking ferrous glycinate with meals lowers side effects but can slow improvement if iron stores are critically low.
Uncommon Reactions
A less talked about side effect pops up every time someone goes to the bathroom: black or dark stools. It surprises a lot of folks and sometimes sparks Google searches for scary reasons, but this harmless color change just comes from leftover iron passing through. Though rare, allergic reactions can bring out hives, swelling, or trouble breathing. Any sign of these deserves an emergency visit, since it could point to a serious problem.
Absorption Battles
One tricky part with iron supplements like ferrous glycinate deals with how other daily choices change its effects. Drinking coffee or tea around the same time can block iron, even in the gentle glycinate form. Calcium—found in milk or cheese—acts as a bouncer, keeping iron out. On the opposite note, vitamin C from orange juice or strawberries helps iron sneak in. Some folks chasing better energy sometimes toss all these supplements together, hoping for a shortcut. It’s easy to forget that too much iron can overload the body, building up in the liver and other organs. High doses for long periods can trigger headaches, joint pain, or problems in people with hemochromatosis, a genetic issue where iron builds up way too fast.
Keeping It Safe
Ferrous glycinate helps many get their strength back, but it’s not a “set it and forget it” fix. Routine check-ups and tracking lab results stop an iron boost from turning into an iron overload. Healthcare providers give advice based on each person’s diet, age, medical history, and how bad the deficiency looks. No two bodies handle supplements the same way. Real results only show up with open conversations: sharing honest feedback about side effects, asking for alternatives if problems show up, and never taking more than recommended. No shortcut replaces common sense—better iron levels come from a mix of good food, the right supplement, and close attention to any odd changes in how you feel.
How should I take Ferrous Glycinate?
Understanding Ferrous Glycinate’s Role
Iron helps the body make the proteins that carry oxygen, like hemoglobin in blood and myoglobin in muscles. Ferrous glycinate stands out because it’s less harsh on the stomach than other iron supplements. It can help people with low iron levels restore their energy and mental clarity. Many parents turn to this form for their kids, and doctors often suggest it for expectant mothers who need gentler iron.
How to Take It for the Best Results
Taking ferrous glycinate feels simple, but creating a daily habit matters. Most doctors recommend swallowing it with water, away from large meals. The reason? Food—especially dairy, tea, or coffee—keeps the body from absorbing as much iron. Vitamin C, like the kind in orange juice or a few strawberries, can help your body soak up more iron, so pairing a supplement with a glass of juice works well.
Everyone’s body reacts differently. Some people may get a little queasy on an empty stomach, so a small bite of bread or fruit can settle things. Notice any stomach cramping or constipation? Drinking more water and adding fiber from vegetables may help.
Finding the Proper Dose
Dosing isn’t one-size-fits-all. Age, sex, and how low someone’s iron runs all influence the number of milligrams you need. Kids, adults, pregnant women, and those managing chronic illnesses show different needs. Blood tests check your ferritin and hemoglobin. A doctor reviews the results and picks a dose for you, rather than guessing or following internet trends. Too much iron can start to damage organs in the body, so the right dose matters.
Recognizing the Signs of Improvement
Better energy, fewer headaches, and less dizziness—these changes let you know the supplement works. Some people notice their concentration improves. Nail strength, less hair loss, and even skin color shifting from pale to healthy reflect real change.
Seeing these shifts can take weeks, not days. Iron levels build up slowly. Stomach trouble, black stools, or an odd metallic taste are common, but severe pain or vomiting need a doctor’s attention right away.
Choosing a Reliable Product
Supplements aren’t all created equal. Some brands skimp on quality, blend in fillers, or mislabel the actual amount of iron. Check for certifications, seek input from a pharmacist or your family doctor, and dig around for reviews from other real people.
Solutions for Better Outcomes
For people who cannot swallow pills, some companies offer liquid or chewable versions. These taste better and help kids or adults with swallowing problems stay on track.
Building a routine—leaving your iron supplement next to your toothbrush or setting an alarm—means you won’t forget a dose. Tracking your progress with a journal or app reminds you why you started and helps your doctor see if adjustments are needed.
Iron supplements make a real difference for people struggling with fatigue, shortness of breath, and trouble focusing from anemia. Consistent dosing, the right pairing with food or juice, and regular check-ins with a physician keep your health moving in the right direction.
Is Ferrous Glycinate safe during pregnancy?
Understanding Ferrous Glycinate and Pregnancy Nutrients
Pregnancy brings a shopping list of questions about what’s safe for mother and baby. Friends might offer advice on spinach, liver, and the strange cravings that pop up in the middle of the night. Prenatal nutrition isn’t just about cravings. Iron stands out as one of the must-have elements for a healthy pregnancy, given the risk of iron-deficiency anemia as blood volume rises. Ferrous glycinate shows up on supplement shelves with soft promises of gentle digestion and solid absorption.
Why Iron Matters for Mothers-to-Be
Iron fuels hemoglobin, the part of red blood cells that moves oxygen throughout the body. Pregnancy asks the body to step up blood production to support both the mother and the growing fetus. Low iron makes many women feel restless, tired, or dizzy. Severe deficiency raises the risk of early delivery and low birth weight. The CDC and the World Health Organization both recommend iron supplements for pregnant women who can’t hit the mark through food alone.
How Ferrous Glycinate Stands Apart From Other Iron Forms
Not all iron supplements play by the same rules. Ferrous sulfate pills often get handed out, but many women tell the same story: stomach aches, constipation, and a lingering taste that’s hard to shake. Ferrous glycinate mixes iron with the amino acid glycine, which helps the body pull in iron with less stomach upset. Few people want to stick with a supplement that makes life harder. In my own practice, I’ve seen women stick to ferrous glycinate longer than other iron pills, because the side effects shrink and daily routines don’t get off track.
Science Backs Up Its Safety for Mothers and Babies
Studies published in journals like the Journal of Obstetrics and Gynaecology Research and European Journal of Clinical Nutrition support ferrous glycinate’s safety for pregnant women. These trials follow groups of women through pregnancy, comparing ferrous glycinate to other standard iron forms. The findings spot less stomach discomfort, similar or better increases in hemoglobin, and no signs of harm to the fetus. Regulatory agencies in the US and Europe recognize ferrous glycinate as safe, when used as directed.
Potential Side Effects and Considerations
While side effects show up less often, nobody wants surprise symptoms. Some women still notice mild constipation or nausea. Overdosing brings risks like with any iron supplement — too much iron can stress the liver or cause digestive trouble. Always wise to check dosage with a doctor, especially if other supplements are in play. Vitamin C can help the body soak up more iron, so adding orange juice or strawberries with a morning dose can give absorption a boost.
Paths to Safer Supplement Choices
Every pregnancy differs, shaped by genetics, health conditions, and diet. Blood tests help zero in on how much iron each woman really needs. Doctors often pick supplements based on how well each patient tolerates them, not just the brand or claims on the bottle. Reading labels with FDA approval and looking for trusted brands can keep risky additives out of the equation. Pharmacists and registered dietitians offer another layer of support, sifting through options that fit an individual’s health plan and pocket.
Building Trust With Science and Professional Advice
Pregnancy asks parents-to-be to sort through a jungle of health claims and opinions. Trust takes root in clear communication between patient and health professional backed by science, not hype. Ferrous glycinate feels like a simple, practical choice for many women who need iron without harsher side effects. The real key remains listening to the body, watching lab results, and keeping up with appointments to track progress every step of the way.
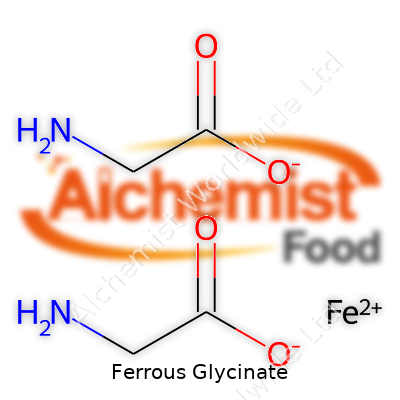
| Names | |
| Preferred IUPAC name | Iron(2+) bis(glycinate) |
| Other names |
Ferroglycine Glycinate Iron(II) Iron(II) glycinate Iron bisglycinate Glycine, ferrous complex Ferrous bisglycinate |
| Pronunciation | /ˈfɛr.əs glaɪˈsɪn.eɪt/ |
| Preferred IUPAC name | Iron(2+) bis(glycinate) |
| Other names |
Ferrous Bisglycinate Iron Bisglycinate Glycine Chelated Iron Ferroglycine |
| Pronunciation | /ˈfɛr.əs ɡlaɪ.sɪ.neɪt/ |
| Identifiers | |
| CAS Number | 20150-34-9 |
| Beilstein Reference | 1907377 |
| ChEBI | CHEBI:31538 |
| ChEMBL | CHEMBL2105969 |
| ChemSpider | 61375 |
| DrugBank | DB09297 |
| ECHA InfoCard | 03b0e8fabd53-44be-aac4-7d9b8806cad7 |
| EC Number | [“27415-87-2”] |
| Gmelin Reference | 77882 |
| KEGG | C18602 |
| MeSH | D018128 |
| PubChem CID | 14798 |
| RTECS number | MB5800000 |
| UNII | D3O2L58H4S |
| UN number | UN3077 |
| CAS Number | 20150-34-9 |
| Beilstein Reference | 3857814 |
| ChEBI | CHEBI:74978 |
| ChEMBL | CHEMBL2104077 |
| ChemSpider | 88164 |
| DrugBank | DB13136 |
| ECHA InfoCard | ECHA InfoCard: 100.111.447 |
| EC Number | [“237-665-9”] |
| Gmelin Reference | 77886 |
| KEGG | C2095 |
| MeSH | D018719 |
| PubChem CID | 16219112 |
| RTECS number | MB8600000 |
| UNII | 9IP20MUD2B |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C4H8FeN2O4 |
| Molar mass | 204.95 g/mol |
| Appearance | Light yellow or off-white crystalline powder |
| Odor | Odorless |
| Density | 0.7 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.72 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.9 |
| Basicity (pKb) | 8.83 |
| Magnetic susceptibility (χ) | Paramagnetic |
| Dipole moment | 4.49 D |
| Chemical formula | C4H8FeN2O4 |
| Molar mass | 204.00 g/mol |
| Appearance | Light yellow or off-white powder |
| Odor | Odorless |
| Density | Density: 0.6 g/cm³ |
| Solubility in water | Soluble in water |
| log P | “-1.32” |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.7 |
| Basicity (pKb) | 8.80 |
| Magnetic susceptibility (χ) | Paramagnetic |
| Dipole moment | 2.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 171.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1510.68 kJ/mol |
| Std molar entropy (S⦵298) | 373.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1519.4 kJ/mol |
| Pharmacology | |
| ATC code | B03AA10 |
| ATC code | B03AA10 |
| Hazards | |
| Main hazards | May cause irritation to eyes, skin, and respiratory tract. |
| GHS labelling | GHS07, Warning, H315, H319, H335 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: If medical advice is needed, have product container or label at hand. Keep out of reach of children. Read label before use. |
| NFPA 704 (fire diamond) | 1-1-0-N |
| Lethal dose or concentration | LD50 (oral, rat): 1,240 mg/kg |
| LD50 (median dose) | 1,350 mg/kg (rat, oral) |
| NIOSH | MW4000000 |
| PEL (Permissible) | 30 mg/kg |
| REL (Recommended) | 30 mg elemental iron |
| Main hazards | May cause irritation to eyes, skin, and respiratory tract. Harmful if swallowed. |
| GHS labelling | **"GHS07, Warning, H302, P264, P270, P301+P312, P330"** |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No Hazard Statements. |
| Precautionary statements | Precautionary statements: "If medical advice is needed, have product container or label at hand. Keep out of reach of children. Read label before use. |
| NFPA 704 (fire diamond) | 1-0-0-N |
| Lethal dose or concentration | LD50 (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | 1200 mg/kg (rat, oral) |
| NIOSH | NO8750000 |
| PEL (Permissible) | 15 mg/kg |
| REL (Recommended) | 30 mg |
| Related compounds | |
| Related compounds |
Ferrous Gluconate Ferrous Fumarate Ferrous Sulfate Ferric Citrate Ferric Ammonium Citrate Ferrous Ascorbate Iron(III) Glycinate |
| Related compounds |
Ferrous bisglycinate Ferrous sulfate Ferrous fumarate Ferrous gluconate Iron(II) glycinate Glycine Iron(II) sulfate |

