Ferrous Citrate: Unearthing Its Journey and Impact
Historical Development
Ferrous citrate has roots reaching back to the rise of mineral supplements in the 19th century. As physicians and chemists looked for better ways to address iron-deficiency anemia, citric acid's chelating powers grabbed attention. Early developers saw that citric acid formed a more bioavailable compound with iron, less constipating and gentler than older iron salts. It didn't take long for supplement makers to recognize one of its assets: people could absorb the iron without most of the gastric distress that chased away patients from iron sulfate or gluconate. Over the decades, research into iron deficiency kept driving demand for clean, reliably absorbed sources of the metal. Ferrous citrate found a place in vitamin tablets and powders, soon spreading into clinical use and food fortification. Each step in its history brings together ambition to fight blood disorders and practical need to create iron compounds the body can handle.
Product Overview
Ferrous citrate sits on shelves as a supplement, packaged in capsules and tablets, sometimes added to powdered drink mixes or infant formulas. Its appeal among dieticians and formulators comes from blending benefits of iron and citric acid. On the consumer side, it offers a non-chalky mouthfeel and rarely produces the metallic aftertaste that haunts other iron pills. Supplement shoppers, especially women and those with restricted diets, gravitate toward it as a versatile answer to low iron levels. Pharmacies and online stores stock versions derived from both food-grade and pharmaceutical-grade manufacturing. Quality standards differ among brands, but a focus on reliable absorption is one constant across the industry.
Physical & Chemical Properties
Looking at a heap of ferrous citrate powder, one finds a pale greenish or faintly yellow material, fine and easy to disperse. Its solubility in water is modest, though heat and acid can encourage more iron into solution. The compound forms as a coordination complex – ferrous ions locked to citrate – which resists quick oxidation but still carries a risk of turning ferric if left damp or heated during storage. On a molecular scale, the iron sits at the core, geometrically surrounded by three-pronged citrate ligands. This structure matters: it resists clumping and allows the molecule to travel into the gut more smoothly than some crystalline forms of iron. The molecular weight, roughly 245.95 g/mol, and an iron content typically around 20-24%, give this compound its economic and nutritional punch.
Technical Specifications & Labeling
Labeling and sale of ferrous citrate products follow strict government and industry rules. Ingredient lists clearly state “ferrous citrate,” and legitimate bottles display measured iron amounts per dose. US and EU pharmacopeias set minimum and maximum acceptable limits for lead, arsenic, and microbe levels. FDA and EFSA regulations demand batch testing to check for impurities and to confirm elemental iron content matches label claims. Most products indicate storage instructions, generally calling for a cool, dry, airtight location. Out-of-spec material, particularly with off-grade color, excess oxidation, or excessive heavy metals, gets rejected during quality control checks. On finished goods, health warnings flag possible side effects such as nausea, constipation, and the risk of accidental overdose among children, echoing the CDC’s emphasis on poisoning prevention.
Preparation Method
Manufacturers produce ferrous citrate through a reaction of iron(II) salts and citric acid. Iron(II) sulfate acts as a popular starting material, dissolved and then treated with an aqueous solution of citric acid under controlled, oxygen-poor conditions to ward off unwanted oxidation. Temperature, stirring speed, and pH all influence the process. As the solution mixes, ferrous citrate gradually precipitates, collected by filtration or centrifugation. To secure reliable purity, producers wash the precipitate, often with cold water or a dilute acid, and then dry it under vacuum or at low temperatures out of sunlight. Operations keep the product away from air and moisture at every step, knowing how quickly ferrous iron can turn rusty when mishandled. Scale-up to industrial levels brings its own headaches—managing waste streams, reusing process water, and controlling byproducts, often citric acid residues, all factor into the plant's success.
Chemical Reactions & Modifications
Ferrous citrate stays mostly stable at controlled pH and in sealed containers, but it does not like exposure to excess heat, light, or air. In solution, it can participate in classic redox chemistry, with ferrous ions oxidizing to ferric states under the right conditions. Aged solutions may produce brown ferric impurities. Formulators sometimes adjust the iron-to-citrate ratio, giving rise to basic and neutral salts, each with slightly different solubility and stability. Some food scientists try coating ferrous citrate particles with lipids or binders to delay oxidation and mask the taste in sensitive retail products. Chemical modifications usually seek improved shelf life or better handling in vitamin blends and liquid suspensions.
Synonyms & Product Names
Pharma catalogs and scientific literature list ferrous citrate under titles like iron(II) citrate, ferroso-citrate, and EINECS code 236-613-0. Commercial powders may show names such as food-grade iron citrate, dietary iron citrate, or even “gentle iron” in consumer marketing. Academic articles sometimes use older designations, reflecting changes in nomenclature over time. Knowing the synonyms helps researchers navigate both regulatory filings and worldwide patent databases where naming conventions don't always match.
Safety & Operational Standards
Sustained safety practices define iron supplement manufacturing. Production workers watch for dust exposure, keeping skin and eyes shielded. Air quality keeps within the limits set by OSHA and corresponding local authorities — even routine cleaning targets iron and acid spills that could build up on factory floors. Shipping regulations mandate leakproof, sealed packaging to avoid both physical contamination and iron oxidation en route to stores or clinics. Health care professionals caution users about both acute and chronic iron toxicity, focusing on accidental overdose in children, risk during pregnancy, and possible interactions with drugs like tetracycline. Shelf packaging and instruction inserts repeat these warnings, often reminding buyers to keep products out of reach from young children. Product recalls remain rare but usually trace back to either excessive heavy metals detected or adulteration issues from poorly controlled supply chains.
Application Area
Dietary supplements draw the most attention, as ferrous citrate gets slotted into daily iron tablets, multivitamins, and prenatal formulas. Medical professionals turn to it in injectable forms for rapid repletion where oral dosing won’t work. Food technologists fortify cereals, flours, and dairy mimics, searching for iron sources that don’t alter taste or shelf life. Animal nutritionists also rely on the compound to patch up iron levels in piglets and poultry, giving livestock a healthier start and cutting losses from anemia. Outside the nutrition sphere, there are smaller markets in laboratory reagents, plant tissue culture media, and catalysts for specific organic syntheses. R&D labs explore new uses, focusing on the chelating property to mop up excess metals in industrial wastewater.
Research & Development
Current research expands on ways to optimize iron absorption and tackle the twin challenges of palatability and gastrointestinal tolerance. Academic and industrial groups investigate ways to tweak crystal structure or partner the compound with synergistic nutrients like vitamin C. Some projects investigate encapsulation, where micro-particle coatings delay iron’s release until it clears the stomach's harsh acid. Other studies inch toward using ferrous citrate as a carrier for other drugs, exploiting the chelate's ability to unlock iron even in hostile digestive tracts. New studies examine whether genetically sensitive populations might draw special benefit from these forms compared to ancient iron sulfates.
Toxicity Research
Toxicologists put the compound under close scrutiny. Acute iron toxicity presents the most serious risk, especially among young children drawn to the candy-like tablets. Ingesting several grams leads to vomiting, abdominal pain, and — in severe cases — life-threatening organ damage due to free radical injury. Chronic intake at levels above recommended daily allowance accumulates in the liver and causes iron overload syndromes, including hemochromatosis. Researchers measure LD50 values, run sub-chronic feeding trials in rodents, and collect real-world incident reports to drive tighter labeling and packaging laws. Manufacturers limit permissible contamination and check each batch for trace metals, including lead and mercury, to avoid compounding toxicity risks.
Future Prospects
Ferrous citrate holds out promise for next-generation nutrition and therapeutics. With the growth of veganism and the spread of “clean label” foods, demands for better iron solutions only swell. Researchers bet on advanced delivery systems that can sideline taste issues and pair iron with other micronutrients. Environmental goals nudge manufacturing toward greener, zero-waste processes and highlight the value of recovering iron from industrial byproducts. Policymakers and public health experts look toward tailored dosing in populations at highest risk for anemia, with computer modeling and blood-monitoring apps streamlining supplement regimens. Novel diagnostic approaches may soon match the right compound to the right patient, cutting overdose rates and making sure supplements finally deliver on decades of research. The drive for better forms of iron remains strong, blending materials science with nutrition to close the global iron gap.
What is Ferrous Citrate used for?
Why Ferrous Citrate Matters
Growing up, people in my family always talked about feeling tired and low on energy, especially my grandmother. The doctor once told her she had anemia, and that she lacked enough iron in her blood. It’s no secret that iron feeds life; your body builds red blood cells using iron, which ferries oxygen all over. Like a city running low on buses, your body gets sluggish when it doesn’t have enough of this mineral.
Ferrous citrate steps onto this stage as a type of iron salt that doctors recommend to boost iron levels. It’s used mainly to treat or prevent low iron conditions like anemia. Unlike some forms of iron, ferrous citrate tends to land a bit softer on the stomach. Busy adults, picky kids, or seniors with fragile digestion all need options that won’t upset their bellies. Iron’s essential, but nobody needs the added misery of nausea just for the sake of a pill.
What Sets Ferrous Citrate Apart?
Some iron supplements hit hard. Folks talk about chalky tablets or hard-to-swallow capsules that leave a metallic aftertaste. Ferrous citrate, used in pharmacies and health stores, comes with a bit of an edge. Chemically, it combines ferric iron and citric acid. This pairing helps make the iron easier for your gut to take in. For people whose bodies struggle to absorb nutrients, this can mean finally seeing results after months of trying.
Absorption matters. So does tolerance. Plenty of iron pills leave folks dealing with constipation or black stools. I’ve met busy parents dropping iron supplements for this reason alone—nobody wants extra hassle in the bathroom. Ferrous citrate sometimes causes fewer of these issues, though everybody reacts differently. Doctors keep this in mind and often suggest ferrous citrate to patients who couldn’t stick with other iron forms.
Facts and Safety
Treating iron deficiency isn’t about guessing. According to the World Health Organization, iron deficiency anemia is one of the world’s top nutritional problems. Kids and pregnant women face the greatest risk. Left unchecked, iron deficiency can hurt mental development, cut down exercise endurance, and sap the immune system’s fight. Over the years, researchers compared various iron formulations. They found that forms like ferrous citrate could fit better for those who need steady, gentle supplementation.
Like anything, misuse causes trouble. Taking iron you don’t need leads to stomach pain, vomiting, or worse. Children especially face higher danger from accidental overdose. That’s why any iron supplement—including ferrous citrate—should be taken with a doctor involved. Trusting your instincts or following internet trends can backfire. Testing iron levels and tracking symptoms works far better.
Solutions and Steps Forward
Doctors and pharmacists have a role in making sure the right person gets the right kind of iron. Detailed conversations, targeted blood tests, and thorough follow-ups make all the difference. Health educators need to explain not just the “what,” but the “why” behind iron therapy. Community clinics need access to affordable, easy-to-tolerate iron sources like ferrous citrate. Simple answers rarely fit complicated lives, but options like ferrous citrate mean more people get what they need.
Iron builds successful days, sharp thinking, and strong bodies. By choosing the right form, like ferrous citrate, health providers support more folks on their way to better health.
What are the potential side effects of Ferrous Citrate?
Understanding Ferrous Citrate
Ferrous citrate shows up in a lot of iron supplements found in local pharmacies and grocery stores. This type of iron often gets recommended for people who deal with iron deficiency anemia or those who just need to bump up their iron intake. Many of us have turned to iron in some form at one point—maybe after a bad bout of fatigue, pregnancy, or a doctor’s warning about low hemoglobin.
Common Side Effects That Aren’t so Fun
Stomach trouble seems almost baked into the deal with iron pills. Ferrous citrate can stir up some unwelcome digestive changes. Constipation takes the top spot for frustration, especially if you’re already dealing with a sluggish digestive system. Diarrhea hangs around as another irritating possibility, which seems unfair after signing up for something that's supposed to help.
Gastrointestinal discomfort, like cramping, nausea, or a sour stomach, happens more often than people might expect. A meta-analysis from the American Journal of Medicine put the rate of GI side effects for oral iron at up to 70% in some studies. Some folks try to get around this by taking their tablets with food, but that runs the risk of lowering how much iron actually gets absorbed.
Dark stools commonly follow iron supplements. The first time I saw it, I thought something was seriously wrong before realizing it’s normal. But black stools can sometimes hide a true problem, like GI bleeding, so staying alert to new or severe changes matters.
Allergic Reactions and Less Common Outcomes
Rashes, swelling, or itching rarely connect themselves to ferrous citrate, but they do pop up in some people. Shortness of breath or swelling of the face could signal a real emergency—something to address immediately rather than trying to ride it out. Having a conversation with a healthcare provider about new symptoms seems like common sense, but many try to tough it out.
Iron supplements can interact with other meds, too. People taking thyroid medicine, antibiotics, or medications for heartburn might see their other medicine stop working as well. This isn’t always explained at the pharmacy, so reading up or asking directly helps avoid surprises.
Long-Term Effects and Hidden Risks
Most people don’t stay on iron forever, but some chronic health problems call for ongoing supplementation. Iron overload can sneak in during this process. Extra iron may gather up in the liver, heart, or pancreas, raising the risk for problems like liver disease or diabetes down the road. A blood test that checks ferritin levels makes sure your system’s not drowning in iron.
In my own family, a relative with hereditary hemochromatosis discovered she was taking extra iron she didn’t need. Her primary care doctor caught it before damage happened, but regular blood work spared her from years of silent harm.
What Can Be Done About Side Effects?
Taking iron supplements with a little food helps many people steer clear of cramps or nausea, though taking them on an empty stomach boosts absorption. Splitting the dose or switching the time of day offers another fix. Sticking with lower doses for longer time spans sometimes gets the right effect without the big side effects.
Working with a healthcare professional instead of buying just any supplement seems like a smart idea. Doctors can check underlying causes for low iron and make sure you actually need it before starting up. They also look for food-based solutions to keep you stocked with iron through leafy greens, beans, and meat instead of reaching for pills all the time.
Iron helps people bounce back from tiredness and gets them through busy days. Choosing to take ferrous citrate without understanding the side effects might trade one problem for another. Staying informed and looking out for changes in your body goes further than just reading a bottle label.
How should Ferrous Citrate be taken or dosed?
The Basics of Ferrous Citrate
Taking iron isn’t always simple. Ferrous citrate, a form of iron, turns up in supplement aisles for folks who struggle with low levels of iron or iron-deficiency anemia. The body needs iron to make hemoglobin—so if you skip iron, your energy skips out on you. Coffee withdrawals have nothing on iron-deficiency fatigue.
Figuring Out the Dose
Determining any iron supplement dose takes more than peeking at a label. Doctors typically recommend between 50 to 100 milligrams of elemental iron for adults each day to treat anemia, but the specific number shifts based on blood test results and age. For children, dosing shrinks—too much iron can hurt developing organs.
Ferrous citrate doesn’t give you all elemental iron. A 100-milligram tablet contains less elemental iron than it sounds. People often misunderstand the conversion. For example, if you read "Ferrous Citrate 100 mg," you’re not getting 100 mg of usable iron; you’re getting about 24 mg of the stuff your blood uses. Always check the label for "elemental iron," not just the total compound weight.
Timing Matters
Taking iron on an empty stomach usually boosts absorption, but that comes with tradeoffs. Empty stomach iron can kick up nausea or cramps. People I know, myself included, have found that a glass of orange juice chases the pills down easier. Vitamin C helps your body grab more iron and eases some discomfort. Dairy, coffee, and tea block absorption. I once washed down my iron with a morning latte for weeks—not the smartest move, since it wasted the pills.
The Food and Medicine Dance
Certain foods and medications mess with iron. Calcium-heavy foods, like cheese or yogurt, slow down iron’s journey through your gut. Medications like antacids or thyroid pills can stop you from getting enough iron, too. Spacing out doses by two hours keeps everything working better. I learned this the hard way, dealing with thyroid meds and iron at the same time—not a great combo.
Checking for Side Effects
No one talks about iron’s effects on your stomach until it’s too late. Constipation or stomach aches show up fairly often. Drinking more water or eating foods with fiber can help. If side effects go beyond discomfort and turn serious, toss the idea of handling it yourself and discuss it with a doctor.
Taking Ferrous Citrate Safely
Supplements aren’t one-size-fits-all. Getting bloodwork before starting any iron supplement sheds light on what your body actually needs. Overdosing on iron isn’t harmless; too much builds up, damaging organs over time. As tempting as it can be to skip appointments and self-treat with over-the-counter iron, this usually leads to more harm than good. If you ever notice dark stools or abdominal pain, it signals a need to talk with a healthcare provider.
People using iron long-term or in high doses: regular follow-ups and checking ferritin or hemoglobin levels make all the difference. Sticking to the right dose, keeping an eye on food interactions, and choosing the right time of day—it all comes together to make ferrous citrate work for you.
Is Ferrous Citrate safe during pregnancy?
Checking the Label on Iron
Pregnancy brings out a flood of well-meaning advice. A woman’s doctor hands her a list of things to avoid, things to eat more of, and supplements that claim to help the baby grow healthy and strong. Iron ranks high on this list since iron-deficiency anemia affects nearly 15% of pregnant women in developed countries. There’s a real question many women face: is ferrous citrate a safe choice for getting enough iron during those busy months?
Reading Beyond the Bottle
Ferrous citrate pops up on pharmacy shelves alongside more recognizable names like ferrous sulfate or ferrous gluconate. Its job is simple — deliver iron in a form the body can use to help produce red blood cells for mom and growing baby. As someone who’s spent plenty of time combing through supplement labels during my own family’s pregnancies, it’s easy to get lost in the chemistry. Most pregnant women have heard about iron’s importance but rarely encounter words like “citrate” outside of a science classroom.
Ferrous citrate is basically an iron salt, and its main draw often comes down to fewer stomach complaints compared to other forms. That’s a practical angle for many expectant mothers already battling morning sickness and heartburn. Studies suggest the body absorbs many iron salts at similar rates, including citrate. The crucial detail is how much elemental iron each tablet delivers and how well an individual tolerates it.
Real Safety Takes a Personal Approach
The U.S. National Institutes of Health (NIH) lists iron as an essential mineral during pregnancy, with daily needs rising to almost double the usual amount. Prenatal vitamins bridge the gap for many people. Not all iron supplements cause the dreaded constipation or upset stomach, but the risk varies. Clinical trials and medical organizations give a green light for iron supplementation for pregnant people, and ferrous citrate doesn’t stand out as riskier compared to other forms.
Every supplement, even those available over the counter, deserves respect. Too much iron can bring headaches, nausea, or more serious challenges, especially if someone mistakenly takes several products at once. That’s why I always recommend checking with a healthcare provider before starting anything new, especially during pregnancy. A doctor can run a quick blood test, check for anemia, and pick the right dose. This avoids both deficiency and the accidental build-up of excess iron, which rarely helps anyone.
The Bigger Picture: Nutrition and Absorption
Iron from supplements works best alongside good nutrition. Vitamin C helps the body use iron better, so a cup of orange juice or some fresh fruit with that morning tablet can help. Certain foods and drinks, like coffee, black tea, or calcium-rich dairy, can slow down absorption. So, a little meal planning pays off. I’ve seen moms improve their iron levels just by tweaking breakfast habits rather than jumping straight to the strongest or latest supplement.
Practical Choices for Expectant Mothers
For pregnant people worried about iron, ferrous citrate offers a useful option. It sits next to other safe choices, and for those who can’t stomach the standard ferrous sulfate, it may mean fewer digestive troubles. Still, not every woman needs a high dose, and some may get enough iron from a prenatal vitamin and their diet. Listen to medical advice, know what’s in each supplement, and stay honest about side effects like nausea or constipation. What matters most is meeting iron needs in a way that feels manageable day to day. Iron supports healthy brain development and strong blood supply for both mother and child. For that reason, every pregnant woman deserves clear guidance on how to reach — but not overshoot — her iron goals.
Can Ferrous Citrate interact with other medications?
Why Taking a New Supplement Calls for a Closer Look
Walking through any pharmacy aisle, it's tough to ignore the lineup of iron supplements promising to fix tired days and cranky moods. Ferrous citrate keeps popping up on those shelves, pushing itself as a more digestible iron option. But, like most supplements, this one brings friends—unwanted drug interactions that can cause more trouble than it solves. My family has tried countless vitamins, and our doctor’s advice sticks with me: don’t tinker with minerals like iron without knowing what else you’re taking.
Interactions That Matter in Everyday Life
Mixing ferrous citrate with common prescriptions can spell real problems. Let’s talk antibiotics first—tetracycline and ciprofloxacin, for example. They lose their punch when iron hops on board. The iron in ferrous citrate teams up with these drugs in your gut and forms a compound your body just flushes out. Studies from the Journal of Clinical Pharmacology back this up, showing antibiotic levels in the blood fall under the needed threshold if taken with iron.
Then, there are antacids. Mixing antacids for heartburn with ferrous citrate cuts down the absorption of the iron. Calcium and magnesium inside those chewables aren’t just soothing your stomach—they’re also hitching a ride with the iron so your body never sees it. This explains why people with chronic heartburn or people on reflux medications complain that their anemia doesn’t get better, even with iron supplements.
Diabetes brings another twist. Metformin is a mainstay for many, and it turns out that iron supplements can make the medicine less effective by altering gut absorption. This is an area still under research, but findings from the American Diabetes Association have started to raise alarms. For people trying to keep blood sugar in check, one small change like adding an iron supplement can throw off the balance.
How to Protect Yourself from Harmful Mixes
Tossing new supplements into a medicine cabinet isn’t a harmless move. Doctors often warn: keep a list of every pill, vitamin, and supplement, and bring it to every checkup. Even if you buy something over-the-counter, pharmacists need to know, especially if you’re juggling several prescriptions. I once saw my own mother’s blood pressure spiral because her iron supplement reacted with her heart medication—something nobody caught until a savvy pharmacist made the connection.
Spacing out doses can help. Many experts suggest taking ferrous citrate at least two hours apart from antibiotics, thyroid medicines, or antacids to dodge these common problems. Still, that’s only part of the picture. Individual responses can vary, and the timing trick doesn’t always cover every possibility. People living with chronic conditions—especially kidney disease or diabetes—need even more personalized advice.
Better Habits Mean Fewer Surprises
Education changes outcomes. Not everybody has the time to research drug interactions, so clear conversations with health professionals matter more than ever. I’ve learned from experience that quick phone check-ins with the local pharmacy go a long way. Safety information published in the Annals of Internal Medicine agrees, pointing out that patients who talk openly about their supplements have far fewer emergency room visits related to drug mix-ups.
A new supplement like ferrous citrate looks harmless, but it can stir up issues nobody sees coming. Taking a closer look, checking in with professionals, and keeping those medication lists updated—these steps protect everyone in an unpredictable world of prescriptions and pills.
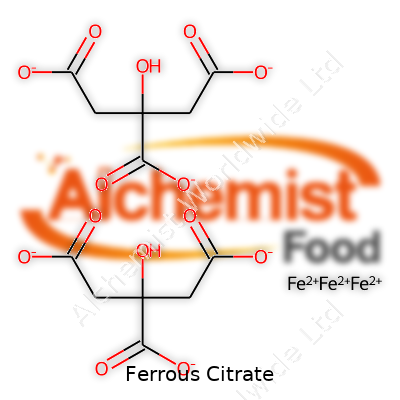
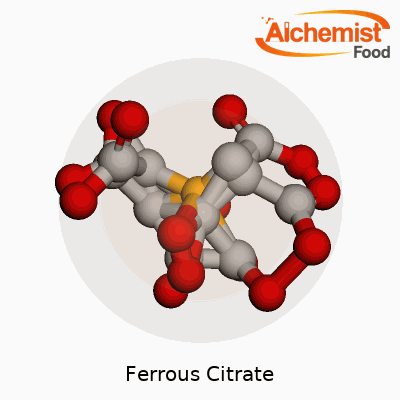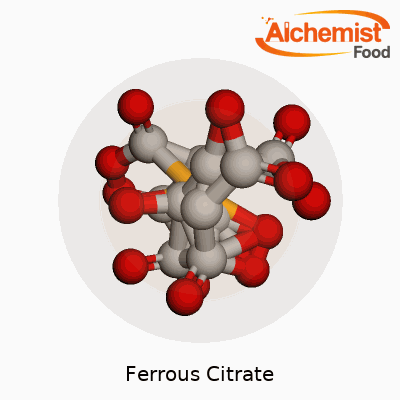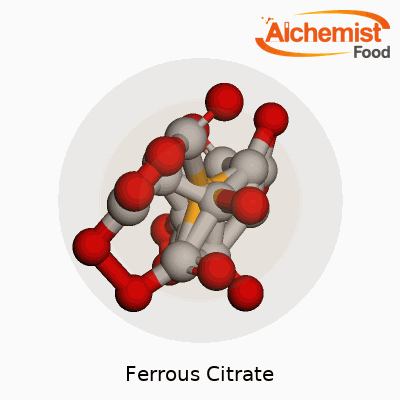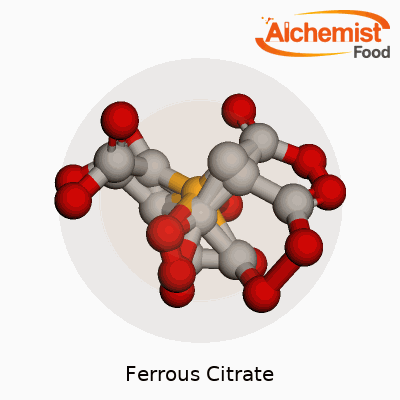
| Names | |
| Preferred IUPAC name | Iron(2+) 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Iron(II) citrate Ferrous dihydrogen citrate Iron citrate Citrate de fer(II) Eisen(II)-citrat |
| Pronunciation | /ˈfɛr.əs ˈsɪ.treɪt/ |
| Preferred IUPAC name | iron(2+) 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Ferric dicitrate Iron(3+) citrate Ferric citrate |
| Pronunciation | /fəˈrʌs ˈsɪtrət/ |
| Identifiers | |
| CAS Number | 141-01-5 |
| Beilstein Reference | 1720862 |
| ChEBI | CHEBI:75831 |
| ChEMBL | CHEMBL1201585 |
| ChemSpider | 54885 |
| DrugBank | DB09288 |
| ECHA InfoCard | 100.039.688 |
| EC Number | 212-484-9 |
| Gmelin Reference | Gmelin Reference: **135080** |
| KEGG | C22236 |
| MeSH | D008403 |
| PubChem CID | 6057346 |
| RTECS number | NO7800000 |
| UNII | A5K2FR16BO |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID9052107 |
| CAS Number | [2338-05-8] |
| Beilstein Reference | 1461302 |
| ChEBI | CHEBI:75831 |
| ChEMBL | CHEMBL1201731 |
| ChemSpider | 22947 |
| DrugBank | DB14646 |
| ECHA InfoCard | 17b591b0-4ccc-4a86-8ca8-920fa02fb45e |
| EC Number | 237-202-1 |
| Gmelin Reference | 12004824 |
| KEGG | C18612 |
| MeSH | D017370 |
| PubChem CID | 61386 |
| RTECS number | GF9810000 |
| UNII | 4B9H1Z4BXP |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID10876367 |
| Properties | |
| Chemical formula | C6H5FeO7 |
| Molar mass | 244.94 g/mol |
| Appearance | Reddish brown crystalline powder |
| Odor | Odorless |
| Density | 0.8 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -3.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.13 |
| Basicity (pKb) | 4.9 |
| Magnetic susceptibility (χ) | −49.0×10⁻⁶ cm³/mol |
| Viscosity | Viscous liquid |
| Dipole moment | 7.231 D |
| Chemical formula | C6H6FeO7 |
| Molar mass | 244.97 g/mol |
| Appearance | A reddish-brown powder. |
| Odor | Odorless |
| Density | 1.5 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -2.43 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.13 |
| Basicity (pKb) | 8.1 |
| Magnetic susceptibility (χ) | −26.8×10⁻⁶ cm³/mol |
| Dipole moment | 6.23 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 418.2 J·mol⁻¹·K⁻¹ |
| Std molar entropy (S⦵298) | 418.5 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | B03AA10 |
| ATC code | B03AA10 |
| Hazards | |
| Main hazards | Harmful if swallowed; may cause irritation to skin, eyes, and respiratory tract. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | IF INHALED: Remove person to fresh air and keep comfortable for breathing. IF SWALLOWED: Rinse mouth. Call a POISON CENTER or doctor/physician if you feel unwell. IF ON SKIN: Wash with plenty of water. |
| NFPA 704 (fire diamond) | 1-0-0-X |
| Lethal dose or concentration | LD50 (oral, rat): 1,330 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 1,240 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 30 mg/day |
| IDLH (Immediate danger) | Not Established |
| Main hazards | May cause respiratory and digestive tract irritation; harmful if swallowed. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Wear protective gloves/eye protection. Wash thoroughly after handling. Avoid breathing dust/fume. If swallowed, rinse mouth. If in eyes, rinse cautiously with water for several minutes. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Lethal dose or concentration | LD50 (oral, rat): 1,480 mg/kg |
| LD50 (median dose) | LD50 (median dose) > 5 g/kg (oral, rat) |
| NIOSH | MW8520000 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 6 mg/day |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Iron(II) fumarate Iron(II) gluconate Ferrous sulfate Ferric citrate Iron(III) citrate Iron(II) succinate |
| Related compounds |
Ferric citrate Sodium ferrous citrate Ferrous sulfate Ferrous fumarate Ferrous gluconate |

