Ferric Oxide: A Modern Commentary on an Old Compound
Historical Development
Ferric oxide has a longer story than most of us suspect, showing up almost everywhere humanity has left a mark. Artifacts from prehistoric times sport streaks of this rusty pigment, linking our earliest creative moments to earth and minerals. Art restorers will tell you that red ochre cave paintings stand as a testament—early humans harvested iron-rich earth, ground it by hand, and shaped the first pigments of civilization. Later, blacksmiths and metallurgists in ancient societies discovered that rusty layers on iron could serve as both a bane—causing corrosion—and a boon, providing a source of pigment and even medicinal powders. In the industrial era, chemists every bit as obsessed as Renaissance artists learned to synthesize it on purpose, not just collect it from rocks. Factories in the nineteenth century began producing ferric oxide to meet demand for paints, abrasives, polishing agents, and steel treatments. Now manufacturers tweak particle size and purity at the molecular level, responding to demands that would boggle an ironworker from the Middle Ages.
Product Overview
Ferric oxide, often sold under names like iron(III) oxide, hematite, or red iron oxide, pops up everywhere from pottery shops to auto plants. In the hardware aisle, those red floor tiles owe their color to this mineral. Crafting a Japanese sword or polishing a telescope lens, chances are you’ll run into a fine, deep-red powder labeled as jeweler’s rouge or just red oxide. For researchers, analytical grades come with strict controls for trace metals and moisture. Grades vary by application—painters go for pigment-grade, builders for construction-grade, and electronics folks need ultrafine, high-purity types for magnetic and semiconductor uses.
Physical & Chemical Properties
At room temperature, ferric oxide shows up as a reddish-brown powder or solid with a metallic sheen in chunkier samples. Hematite, the main mineral form, brings an earthy red tone and density hovering around 5.2 g/cm³. Insolubility in water makes it useful for coatings that face rain or humidity. The melting point sits high, above 1,500°C. Add a magnet, and you’ll see weak magnetic properties—nowhere near as ferrous as iron filings, which reflects its position on the chemical spectrum. Chemists peg its composition at Fe₂O₃, with each molecule fitting neatly into a crystalline structure that plays a role in everything from ore classification to magnetic tape production. Oxidation state matters: ferric means iron(III), not iron(II)—so don’t swap it for ferrous oxide, unless you’re looking for a totally different outcome. In the lab, ferric oxide acts as both oxidizing and reducing agent, but most hands-on work revolves around its stability and colorfastness.
Technical Specifications & Labeling
Buy a kilo of red oxide from an industrial supplier, and you’ll notice specs for Fe₂O₃ content (often exceeding 95% for technical work), particle size (measured in microns), moisture level, and pH. Labels also list heavy metal impurities—just a whiff of lead or arsenic can disqualify a batch for food-contact or cosmetic use. Manufacturers test for oil absorption, since paint and rubber industries demand precise pigment loading. In electronics, magnetic grade calls for nanometer-scale particles and details about coercivity and remanence—properties that matter for tapes, cores, and data storage. On site, regulatory documents flag GHS pictograms, safety codes, use restrictions, and batch traceability. Final users watch for lot numbers, which help trace contamination or performance issues back to the source.
Preparation Method
Industrial processes rely on roasting iron-containing minerals, thermal decomposition of iron salts, or even direct precipitation from iron(III) chloride or sulfate solutions. In a factory, iron ore or scrap gets oxidized at high temperature, yielding pure Fe₂O₃ after grinding and sieving. Some labs prefer chemical routes: dissolving iron metal in acid, then exposing to air or hydrogen peroxide to force oxidation. Control over pH and temperature means tight control of particle size, color, and porosity. Homemade pigment-makers often use rust scraped from old iron tools, but commercial-grade powder involves careful washing, filtration, and drying to ensure consistent results. In some modern approaches, nano-structured forms get synthesized using sol-gel techniques, offering finely tuned magnetic or catalytic properties.
Chemical Reactions & Modifications
Heat ferric oxide with hydrogen, carbon monoxide, or even methane, and the result is metallic iron—a step critical in steelmaking. Expose it to acids, and Fe₂O₃ converts to soluble iron salts, used in water treatment or industrial chemistry. Mix it with aluminum powder for a spectacularly exothermic thermite reaction, which welds steel rails or disables munitions in explosive ordnance disposal. Chemical engineers modify the surface of ferric oxide for use as catalysts, pigment carriers, or drug delivery agents. Adding dopants or changing crystal structure can flip it from a pigment to a key component in electronics, battery cathodes, or medical imaging nanoparticles. The chemistry feels simple at first glance, but real-world applications demand precise control at every step.
Synonyms & Product Names
Market labels for ferric oxide can drive a newcomer up the wall. In hardware stores and paint catalogs, it goes by iron(III) oxide, red iron oxide, hematite, pigment red 101, or just rouge. Cosmetics brands sometimes use “CI 77491,” pointing to its Color Index number. Electronics components list “Fe₂O₃” among starting materials for magnetic cores or coatings. Historical records cite “jeweler’s rouge” or “Venetian red,” two names still common for abrasives and pigments. Each synonym comes with expectations—“hematite” implies natural mineral, “synthetic red” suggests tight spec control, and “magnetic oxide” refers to ultrafine, high-purity material.
Safety & Operational Standards
Handling ferric oxide usually means dust control. Breathing fine powder can irritate lungs; chronic exposure may lead to benign siderosis, a form of iron dust accumulation in the lungs. Factories use local exhaust ventilation, dust masks, and containment systems to keep particles out of the air. Safety data sheets flag precautions: avoid creating airborne dust, store in dry areas, wash hands before meals, and never eat or drink in handling areas. The powder isn’t flammable, but thermite mixtures need careful separation from ignition sources. National and international rules—OSHA, REACH, GHS—set limits for occupational exposure and require clear labeling for health hazards. Environmental guidelines discourage dumping large amounts into waterways, since sediment can disrupt aquatic life. Workers in pigment, construction, and electronics sectors often attend mandatory training on proper handling and first aid for exposure incidents.
Application Area
In my line of work, fields from ceramics to electronics pull on ferric oxide every day. Construction crews mix it into concrete and bricks to get rich, weather-stable colors. Artisans grind it into glass and glazes. Polishers reach for jeweler’s rouge to sharpen lenses and beautify metals. Magnetics researchers seek nanograde samples for magnetic tapes, transformers, MRI contrast agents, or lithium-ion battery cathodes. Even farmers employ micronized forms as trace element supplements in soil or livestock feed. Municipal water systems add ferric oxide-based coagulants to clear up drinking water. On a wider scale, geologists use its presence in soil and rocks to map historical climate conditions, since hematite forms under hot, dry weather. Modern environmental engineers explore its use for removing arsenic from groundwater, since its surface chemistry traps contaminants effectively.
Research & Development
Labs worldwide challenge the limits of ferric oxide, searching for finer particles, higher purity, and specialized forms. Surface modification attracts a large share of attention, especially in biomedical fields where coated particles serve as magnetic markers or drug carriers. Electronics giants contract teams to tune properties for data storage or quantum computing; every improvement in purity or shape means longer-lasting tapes, higher-density drives, and more reliable microchips. Green chemistry researchers test ferric oxide as a photocatalyst for water splitting and pollutant breakdown. In energy, engineers stretch its capabilities for new solar cell designs or as part of hydrogen production. Material scientists build composite materials where the toughness of ferric oxide improves scratch resistance or thermal stability in coatings and polymers, always striving for more performance or lower cost.
Toxicity Research
Human bodies need iron, but don’t benefit from breathing or ingesting too much oxide dust. Clinical data show that workplace exposure, mainly in old-school foundries or pigment plants, correlates with benign iron deposition in the lungs, called siderosis; most cases don’t progress to severe disease, but no one wants unnecessary exposure. Toxicologists measure oral LD50s in the thousands of milligrams per kilogram, showing low acute toxicity. Still, chronic or high-level contact can overload iron metabolism and cause health problems for sensitive individuals. In the environment, ferric oxide’s non-solubility protects most ecosystems, but tiny nanoparticles could slip through filters and affect aquatic microfauna. Regulators keep an eye on particle size, toxicity thresholds, and long-term studies, particularly as nanomaterials expand into consumer products.
Future Prospects
Ferric oxide keeps surprising people in science and engineering, finding new niches as technology moves forward. Nano forms attract funding for medical imaging and targeted treatment, opening possibilities in cancer therapy, drug delivery, and rapid diagnostics. Battery developers hope for energy-dense, environmentally friendly storage materials based on iron oxides, potentially shifting lithium limitations. Green energy pushes ferric oxide as a low-cost photocatalyst or a component in hydrogen production, while environmental chemists expand its use in water purification and heavy metal adsorption. As more high-tech industries demand specific particle shapes, sizes, and coatings, companies invest in smarter synthesis, quality control, and green processing methods. Researchers dig into the atom-by-atom structure to unlock next-generation electronics, magnetic sensors, and composite materials. What started as a pigment in clay-caked hands now promises breakthroughs in medicine, energy, and information technology, provided the materials stay safe, sustainable, and accessible.
What is Ferric Oxide used for?
Why Is Ferric Oxide Everywhere?
Ferric oxide, better known by many people as rust, has a way of sneaking up on anything iron touches. For most folks, rust looks like trouble. Old bikes, metal fences, and parked cars all show its mark if left in the rain. Turn to industry and things change. Ferric oxide becomes a staple for making products and pushing technology forward. A lot of my hands-on learning about this material came from painting, repairing, or watching the metalworkers in my family treat corroded tools—yet it surprised me how valuable those orange-red streaks really are once you dig into how they’re used.
Color That Won’t Quit
The paint aisle in any hardware store shows off just how useful ferric oxide can be. This pigment delivers powerful color for everything from playground equipment to barn roofs. Red ochre, that earthy tone in artists’ palettes and natural wall murals, traces right back to this stuff. Manufacturers keep turning to ferric oxide for its durability, its colorfastness, and the fact it comes from abundant iron. Knowing how it holds up to sunlight and won’t fade easily, artists and companies have relied on it for more than a century. Just think about red bricks, roof tiles, or the paint on industrial machinery—all that color usually owes something to ferric oxide.
Steel’s Tough Secret Ingredient
Steel producers use huge amounts of ferric oxide, but not for color. They grab it for smelting. In the process of turning iron ore into steel, ferric oxide acts as a source of iron while purifying the mix. Proper steel production depends on this chemical to strip away contaminants, feeding the demand for sturdy bridges, clean water systems, and safe cars. Data from the World Steel Association shows that global iron ore, including its oxidized forms, underpins all construction and infrastructure growth. Back in my days shadowing a local welder, I saw firsthand that steel’s strength traces right back to how cleanly it’s produced—with ferric oxide always in demand.
Polishing, Batteries, and More
Beyond paints and steel, ferric oxide keeps showing up. Jewelers and opticians rely on it as “jeweler’s rouge” for smoothing glass, sharpening knives, or giving a final polish to gold. I’ve watched a family friend buff an antique ring to a near-glow, using just a pinch of this powder. These practical uses give the material unexpected value, making it a household staple in some trades.
Science labs and battery manufacturers also line up for ferric oxide. Lithium-iron batteries, growing in use for renewable energy and electric cars, call for iron-based chemistry. Ferric oxide steps up again, powering a greener push for modern technology. Research keeps finding new ways to harness it, from environmental cleanup to electronics.
What About Health and the Planet?
Too much dust from mining or processing ferric oxide isn’t great for lungs. Studies have shown long-term exposure to fine iron oxide particles can irritate workers’ respiratory systems. Responsible mining, good ventilation, and regular mask use help keep people safe, following occupational safety recommendations. On the environmental side, recycling old steel and capturing dust before it escapes into the atmosphere both help limit problems while keeping valuable iron in circulation.
Building a Smarter Approach
Ferric oxide’s wide reach can’t be ignored. End users demand products that last. Companies cut costs by reusing scrap iron. Smart policy and better technology can clean up any mess left behind—while making sure the benefits keep rolling in. Every time I see a faded barn or a polished gold ring, I’m reminded that even the simplest materials, when handled right, can fuel progress.
Is Ferric Oxide safe to handle?
Everyday Contact With Ferric Oxide
Ferric oxide, or rust on an old bike chain, barely grabs attention for most people. Factories use it as a pigment in red paints and construction materials. Anyone who gets iron supplements pops it into their mouth without much thought. My own experience with rusty garden tools—just brushing dirt and orange dust off before using—didn’t cross wires of alarm in my mind. This everyday stuff shows up nearly everywhere, even in food coloring and cosmetics.
What Science Tells Us About Ferric Oxide Exposure
Looking past how common it is, some real questions come up about health. Ferric oxide itself isn’t poisonous in small amounts. Once I checked through research papers, it surprised me how many sources say skin contact means almost nothing for an average person. Rubbing soil or red clay on your hands doesn’t make you sick. The U.S. Occupational Safety and Health Administration (OSHA) actually places it on the low end of hazard lists. The American Conference of Governmental Industrial Hygienists assigned a higher limit for airborne iron oxide dust than for loads of other chemical dusts. That tells me most science reviews see most risks only with large, repeated exposure.
Breathing fine dust, though, turns simple use into a different issue. In big amounts—think factory work sanding paints or mixing concretes—iron oxide dust can get trapped in lungs. Workers in old metal plants developed a condition called siderosis from breathing clouds of this stuff for years. Siderosis doesn't lead to bigger health crises the way asbestos or silica does, but heavy doses still scar lung tissue. Rules in many countries require masks or respirators to keep that problem in check. That matters. Most people don’t see those clouds in their homes, but mixing dry pigments at home or sanding metal all afternoon puts more in the air than you might guess.
Protecting Yourself While Handling Ferric Oxide
I always use gloves and a dust mask in the backyard working with powdery materials. A friend of mine, a ceramics teacher, keeps her workroom ventilated and sweeps briskly after glazing red tiles. The lesson from her: habits matter more than complicated rules for most users. Wash hands before a meal, wear long sleeves if you’re mixing powders, and avoid breathing clouds. Kids love making “potions” with soil or rust; I tell them to play outside and wash up after. That covers most risks for casual use.
Regulations and Reliable Sources
Real concern shows up in bulk handling for months or years. Workplace safety agencies set exposure standards after reviewing how many workers actually got sick. The U.S. Food and Drug Administration still lists iron oxides as safe for coloring food, drugs, and cosmetics at approved levels. European regulations reach the same conclusion. I look for those stamps when deciding if a product feels worth my trust, especially with children or food.
Steps Toward Safer Handling
Solutions exist and don’t call for fancy gear in homes or studios. Wetting powdery iron oxide before mixing it in paints or cements keeps dust down. Good ventilation clears the air fast. For big projects, the disposable masks from hardware stores keep dust out of lungs. In jobs where big volumes get used every day, companies supply real respirators and monitor air quality. Laws—and good habits—cover most gaps. No one needs to panic about rust in small doses, but caution pays off when powders get airborne.
What are the storage requirements for Ferric Oxide?
What Ferric Oxide Brings to the Table
Ferric oxide pops up in many walks of life, from pigment in paints to use in welding and beyond. In busy industrial warehouses, dusty school supply closets, and even garages, you could stumble across this rusty red powder. To keep it working safely and at its best, attention falls right onto where and how it’s stored.
Staying Dry Matters
Ferric oxide clumps up if water gets near. Dampness not only messes with its texture, it starts to invite problems with caking and uneven distribution. At home, I’ve seen fine powders like flour or spices turn rock hard if left open on a humid day. Ferric oxide is no different, only with higher stakes—especially in labs or manufacturing floors. To dodge those headaches, smart storage always keeps it in airtight containers, with tight-fitting lids and seals. Businesses often use sealed drums, while smaller users stick with screw-top jars. Right away, a dry place makes everything easier—less mess and easier weighing.
Saying No to Heat and Sunlight
I used to keep spray paint cans in the shed—big mistake when the sun started baking everything. Ferric oxide also likes dark, cool spots, away from sunlight and heat. Exposure to strong light can alter its color and cut its shelf life. Many industrial bosses designate temperature-controlled rooms far from furnaces or machinery, so the powder doesn’t take on extra heat. Those with just a few kilograms often pick a shelf in a pantry or garage, but away from windows or radiators.
Why You Should Keep Ferric Oxide Secure
Much like paint thinner or old batteries, ferric oxide deserves a spot out of reach of pets and kids. Inhaling its fine dust over time has links to respiratory trouble, from labored breathing to chronic illness. I remember reading about factory workers who wore masks just to handle similar powders because repeated exposure can really add up. Proper labeling, high shelves, and locked cabinets go a long way here. Industries lean on locked storage units and regular safety checks.
Keeping It Clean and Separate
Clutter multiplies the chances of messing up. Storing ferric oxide alongside acids or strong chemicals often sparks unwanted reactions. Ferrous metals or organic materials can cause spoiling or even fire. So, most manufacturers choose shelves meant just for oxides and other stable mineral powders. I once stored garden fertilizer next to bleach, and soon discovered the risk isn’t worth it. Even at home, marking shelves or using plastic bins helps prevent mistakes.
Fire Risks and Spill Control
Though ferric oxide itself doesn’t catch fire easily, mixing it with metals or certain chemicals can start problems. Fireproof storage—like steel cabinets—offers peace of mind, particularly where sparks or flames are nearby. I’ve worked in spaces where a fire extinguisher sat by the supply closet, just in case. Lining floors with non-slip mats catches spills and limits dust from spreading, which helps avoid accidents for folks walking by.
Pushing for Better Storage Practices
Many people underestimate how small changes in storage shape safety and performance. For ferric oxide, sturdy labels, regular cleaning schedules, and a climate-controlled environment help cut down accidents. Staff should get updated training and clear checklists, even if the job feels simple. My own experience working with chemicals taught me the value of checklists; that step alone often stops trouble before it starts. With a bit of effort up front, ferric oxide keeps working without causing headaches, and everyone stays safer in the process.
Can Ferric Oxide be used as a pigment in paints?
A Pigment Rooted in History and Everyday Life
Most people spot ferric oxide quickest as rust, that reddish dust on old tools or garden gates. Yet the stuff goes much deeper than corrosion. Over centuries, artists, builders, and tradespeople reached for ferric oxide for color. From cave paintings to industrial coatings, this pigment weaves through creativity and industry alike.
Ferric Oxide’s Core Qualities
Quality paint needs color that sticks, resists fading, and handles the weather. Ferric oxide stands up to these demands. Its earthy reds, oranges, yellows, and deep browns catch the eye for their intensity and warmth. Unlike many synthetic pigments, ferric oxide comes straight from mineral deposits, making it tough and stable. People searching for paint that lasts outdoors or in sunlit rooms often settle on ferric oxide because it resists UV damage, rainfall, and chemical pollution.
Young artists in school and seasoned painters on construction sites both count on this pigment largely for one reason: it keeps color honest year after year. Even big infrastructure—bridges, pipelines—often sport an iron oxide base coat to block corrosion and cover surfaces for decades.
Safety, Environment, and Health: Clear Choices
Ferric oxide brings peace of mind to parents and professionals alike. Unlike some old-school pigments, it leaves out heavy metals such as lead or cadmium, so paint makers can produce safer, less toxic coatings. This sits well with current trends pushing for more eco-friendly and healthier living spaces. Fewer worries about toxins mean makers can craft paints for use in schools, playgrounds, and hospitals without raising eyebrows.
The mining and processing of ferric oxide sometimes draw questions about sustainability. Some companies already seek smarter ways to source and refine these minerals with less impact on landscapes and waterways. Though greener chemistry will always have a road to travel, ferric oxide keeps holding an edge against more polluting options.
Color Richness and Practical Uses
Walk into any paint store, and you’ll spot rich red and brown samples on the wall. These colors rarely rely on synthetic reds or mixes of several dyes. Ferric oxide stands out for ease of mixing and strong tinting—qualities that allow artists and brands to keep costs in check without cutting corners on appearance.
Masons use iron oxide-based pigments in concrete tiles and bricks. Homeowners see it in terracotta flowerpots and garden stepping stones. Paint companies blend it into protective coatings for ships and trains. Children’s crayons and classroom paints gain their depth from these sturdy iron compounds, letting young hands paint without much risk.
Finding Solutions for the Future
Some critics raise the price and energy footprint of mining as hurdles. More recycling from industrial byproducts, such as repurposing rust from steel plants, may stretch the value of ferric oxide while saving resources. Advanced processing—maybe using less water or solar power—could support cleaner pigment manufacture. Both commercial painters and small-batch artists commonly seek products with strong environmental credentials.
Real trust in a pigment comes from its performance and safety, year after year. Ferric oxide keeps earning its spot in the paint world, not just through tradition, but through a grounded record of durability, color strength, and health-conscious safety.
What is the chemical formula of Ferric Oxide?
Ferric Oxide and Its Chemical Identity
Ferric oxide, better known to many as rust, shows up in everyday life more often than folks realize. Its chemical formula is Fe2O3, which means each particle contains two iron atoms and three oxygen atoms. This simple combination creates the deep red-brown powder you see on corroded tools, old pipes, and even rocky landscapes in desert regions. The stuff people scrape off garden furniture in spring isn’t just a sign of decay—it tells a bigger story about chemistry, industry, and even health.
Iron, Oxygen, and a Little Water
Growing up, I watched my grandfather turn rusty nails into shiny new ones using vinegar and a bit of scrubbing. He’d say, “Iron loves air and water, maybe a little too much.” That’s all it takes for iron to pull oxygen from the air and turn into ferric oxide. It’s a natural reaction that’s nearly impossible to avoid unless you coat that iron with paint or keep it bone-dry. This reaction shapes industries and the way buildings get maintained all over the world.
Why Ferric Oxide Matters
Beyond rust stains on concrete, ferric oxide serves as a staple in several fields. It’s a key ingredient in many pigments, giving color to paints, glass, and ceramics. Walk into any art supply store, and you’re likely to find ferric oxide in various shades of red, brown, and yellow, each one mixed into colors artists trust for depth and durability. In steel production, ferric oxide acts as an important raw material in making pure iron through blast furnace processing. The journey from rusty junk to steel beams holding up city skyscrapers begins with this very chemical.
Health and Environmental Impact
Industrial use of ferric oxide raises some real concerns. Inhaling ferric oxide dust on job sites isn’t just unpleasant—it can spark lung irritation over time. The National Institute for Occupational Safety and Health recommends strict controls on airborne particles for folks working around this material all day. Long-term exposure can also signal environmental issues. Look at soil near mining and smelting regions, and it often carries a reddish tint, a visual sign that iron and oxygen have been busy. Waterways can also turn a rusty color from runoff, which can damage aquatic life if left unchecked.
Taking Practical Steps
There’s no flipping an off-switch on ferric oxide, but practical steps matter. Coatings, regular cleaning, and designing equipment for easier maintenance work together to slow its spread. For communities living near heavy industry, smart monitoring and planting erosion-control vegetation help keep soil and water clean. On the health front, investing in strong workplace protections means fewer respiratory problems and a safer working environment. Smarter industrial processes now focus on capturing and recycling ferric oxide, turning what many see as waste into useful materials—all without extra strain on natural resources.
Conclusion
Ferric oxide’s chemical makeup may sound simple, but its role in daily life runs deep. That old saying about rust never sleeping rings true, yet with some effort and smart choices, rust’s story can shift from nuisance to valuable resource.
| Names | |
| Preferred IUPAC name | Iron(III) oxide |
| Other names |
Iron(III) oxide Ferric oxide red Hematite Iron sesquioxide Red iron oxide Diiron trioxide |
| Pronunciation | /ˈfɛr.ɪk ˈɒk.saɪd/ |
| Preferred IUPAC name | Iron(III) oxide |
| Other names |
Ferric oxide Iron(III) oxide Hematite Red iron oxide Iron sesquioxide Iron oxide red |
| Pronunciation | /ˈfɛr.ɪk ˈɒk.saɪd/ |
| Identifiers | |
| CAS Number | 1309-37-1 |
| Beilstein Reference | 13612 |
| ChEBI | CHEBI:30413 |
| ChEMBL | CHEMBL1201141 |
| ChemSpider | 20644016 |
| DrugBank | DB09533 |
| ECHA InfoCard | ECHA InfoCard: 100.028.762 |
| EC Number | 215-168-2 |
| Gmelin Reference | 789 |
| KEGG | C16276 |
| MeSH | D005348 |
| PubChem CID | 518696 |
| RTECS number | NO4565500 |
| UNII | N1K09ZQ99L |
| UN number | UN1376 |
| CompTox Dashboard (EPA) | DTXSID7020182 |
| CAS Number | 1309-37-1 |
| Beilstein Reference | 13610 |
| ChEBI | CHEBI:30413 |
| ChEMBL | CHEMBL1201532 |
| ChemSpider | 14129 |
| DrugBank | DB11097 |
| ECHA InfoCard | 100.097.386 |
| EC Number | 215-168-2 |
| Gmelin Reference | 120622 |
| KEGG | C16525 |
| MeSH | D050875 |
| PubChem CID | 518696 |
| RTECS number | NO4565500 |
| UNII | PYU4ZW4VY1 |
| UN number | UN1376 |
| Properties | |
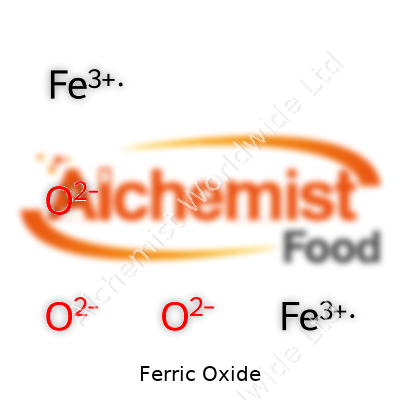
| Chemical formula | Fe2O3 |
| Molar mass | 159.69 g/mol |
| Appearance | Reddish-brown powder |
| Odor | Odorless |
| Density | 5.24 g/cm³ |
| Solubility in water | Insoluble |
| log P | 3.18 |
| Vapor pressure | Negligible |
| Basicity (pKb) | 8.27 |
| Magnetic susceptibility (χ) | 1.9×10⁻³ (SI units) |
| Refractive index (nD) | 1.93 |
| Dipole moment | 0.00 D |
| Chemical formula | Fe2O3 |
| Molar mass | 159.69 g/mol |
| Appearance | Red-brown powder. |
| Odor | Odorless |
| Density | 5.24 g/cm³ |
| Solubility in water | Insoluble |
| log P | 11.08 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 15.6 |
| Basicity (pKb) | 11.42 |
| Magnetic susceptibility (χ) | +1.9×10⁻³ |
| Refractive index (nD) | 2.94 |
| Dipole moment | 0.00 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 87.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –824.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -824.2 kJ/mol |
| Std molar entropy (S⦵298) | 87.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -824.2 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -824 kJ/mol |
| Pharmacology | |
| ATC code | V07BB |
| ATC code | V07BB |
| Hazards | |
| Main hazards | May cause respiratory irritation. May cause mechanical irritation to the eyes, skin, and respiratory tract. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 0, Instability: 0, Special: - |
| Autoignition temperature | 303–430 °C (string) |
| Lethal dose or concentration | LD50 (oral, rat): > 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >10,000 mg/kg (oral, rat) |
| NIOSH | NO7400000 |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | 2500 mg Fe/m³ |
| Main hazards | May cause respiratory irritation, prolonged inhalation may cause lung damage, may cause eye and skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P280, P302+P352, P305+P351+P338, P312 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | 330°C (626°F) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (Oral, Rat): > 5,000 mg/kg |
| LD50 (median dose) | > 30,000 mg/kg (oral, rat) |
| NIOSH | NO4200000 |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | 2500 mg Fe/m3 |
| Related compounds | |
| Related compounds |
Aluminium oxide Chromium(III) oxide Iron(II) oxide Iron(II,III) oxide Manganese(III) oxide |
| Related compounds |
Ferrous oxide Iron(II,III) oxide Iron oxyhydroxide Iron(III) chloride Iron(III) sulfate |

