Ferric Citrate: From Early Chemistry to Modern Applications
Historical Development
Chemists started recognizing the coordination behavior between citric acid and iron long before today’s pharmaceutical standards set in. Ferric citrate first appeared in early 19th-century chemical texts, described as a product formed by mixing iron salts with citric acid. Early pharmacists and apothecaries created their own mixtures with little control over composition or purity, but the health community soon recognized how ferric citrate could help correct iron deficiency. Medical use came as a direct answer to population-wide malnutrition, especially in growing industrial cities. Over decades, tightening controls and developing purification techniques gave ferric citrate a reputation for reliability. Research, consumer advocacy, and tighter regulations have steadily shaped how companies manufacture and label this compound, pushing the field toward better safety, accurate composition, and more deliberate product testing.
Product Overview
Ferric citrate enters the scene most often as a reddish-brown powder or granular compound, used both as a food supplement and as a pharmaceutical. It carries iron in the ferric (III) oxidation state, making it valuable where direct iron supplementation is required. Its reputation in health comes from its dual ability: not only can it raise iron stores, but it also binds and removes phosphate in chronic kidney disease. Some industrial users push it into water treatment—using its metal-binding power to help remove impurities—but medicine tends to dominate the market. Whenever a new ferric citrate batch lands on a manufacturer’s dock, they’re looking for consistent appearance, solubility, and iron content, since these factors determine its value for both pill makers and research scientists.
Physical & Chemical Properties
Whole bags of ferric citrate may leave orange-brown dust on gloves. It dissolves in water, especially at slightly acidic pH, but forms suspended solids in hard basic water. Specific gravity hovers around 1.8—typical of many iron compounds. Chemically, it combines three ferric ions with four, five, or six citrate ligands, resulting in a somewhat variable composition. This flexibility leads to a range of products under similar names, all with the core property of high iron content and moderate solubility. Ferric citrate resists most gentle drying procedures but starts decomposing at high temperatures, a trait users must consider for storage and processing.
Technical Specifications & Labeling
Quality often sits in the details. Commercial ferric citrate typically comes with a certificate of analysis covering iron percentage (usually 16-18%), moisture content, and trace metal levels. Good manufacturers test for heavy metals like lead or arsenic because these pose high risks. Labeling should show not only batch number and expiration date, but precise iron content—patients and doctors rely on these numbers for safe dosage. Tablets and powder packets need tamper-evident packaging, with dosing instructions shaped by clinical trials. Pharmaceutical grades must meet major pharmacopeial standards, such as USP or EP, which ensures doctors and patients get exactly what they expect.
Preparation Method
Making ferric citrate typically starts by dissolving ferric chloride in water and slowly blending in citric acid, keeping pH well controlled. Operators stir and monitor as a reddish precipitate forms, filtering and washing the solid to reduce impurities. In small-scale research or pharmacy settings, this process seems simple, but problems can show up—residual chloride, variable yield, or a batch that doesn’t dry cleanly. Large producers have started shifting to closed systems that recover water, keep iron-to-citrate ratios steady, and remove excess ions. The choice of purification method—often using activated carbon or ion-exchange resins—affects the purity and cost of the final product.
Chemical Reactions & Modifications
Ferric citrate chemistry hinges on the strength of bond formation between iron(III) and citric acid. It readily complexes with other ligands if they enter solution—adding EDTA or oxalate can break it down and form new chelates. Under reducing conditions, iron(III) can convert to iron(II), though citrate slows this process. Some researchers modify ferric citrate with other organics, pressing for specialty applications like slow-release fertilizers or advanced biomedical materials. The stability and behavior of these complexes depend on pH and temperature, so labs monitor reaction conditions closely to avoid product breakdown.
Synonyms & Product Names
Though “Ferric citrate” appears on most chemical bottles, a range of synonyms crowd chemistry catalogs. Names like “Ferric(III) citrate,” “Iron(III) citrate,” and “Citrate of iron” all point back to the same core ingredient, but labeling matters—especially for regulatory review. Pharmaceutical products carry brand names such as Auryxia in the US, used for managing hyperphosphatemia. When buying raw materials, users must double-check both the label and the specification sheet to avoid confusion with less-soluble forms or low-purity analogues.
Safety & Operational Standards
Any time iron compounds come into contact with people, safety becomes central. Ferric citrate rates as slightly hazardous by inhalation and ingestion in raw form, mostly due to the irritation and stomach upset large doses cause. Occupational safety rules require gloves, face protection, and fume hoods during weighing and mixing. Pharmaceutical-grade material has to pass microbiological tests because contaminated iron sources feed dangerous bacteria. Safe storage means cool, dry rooms away from strong oxidizers, acids, or incompatible packaging materials. Hospitals treat accidental overdose with standard iron chelators, and poisoning cases must always be managed with immediate medical oversight.
Application Area
The pharmaceutical world pulls most of the ferric citrate supply; kidney clinics use it to help dialysis patients control blood phosphate, a task nearly impossible without powerful binders. Iron supplementation for anemia touches millions of lives, but a second wave of demand flows from animal feed producers, food supplement makers, and even environmental service companies. Some municipal water utilities experiment with ferric citrate for controlling scale and as a coagulant—a greener alternative to harsher metal salts. Biomedical researchers use it for cell culture media, while the agricultural sector is eyeing slow-release formulations to help crops in iron-poor soils.
Research & Development
Laboratories constantly chase new ways to make or use ferric citrate. Drug delivery researchers test how modified ferric citrate molecules might offer controlled iron release, easing gastrointestinal side effects. Water treatment studies probe deeper into how chelated iron interacts with organic and inorganic pollutants. Major projects explore blending ferric citrate with nanomaterials, hunting for better MRI contrast agents or next-generation iron supplements that sidestep the drawbacks of current products. The data pouring out of universities shows a hunger to understand iron chemistry more deeply, with an eye toward sustainable and safer resource use.
Toxicity Research
No iron supplement escapes scrutiny; research teams study not only acute overdose, but also chronic exposure at low levels. Most human bodies tolerate modest doses—especially when used for short-term medical needs—but high exposure hazards crop up quickly. Children and adults risk iron poisoning primarily through accidental ingestion, with symptoms running from stomach pain to systemic toxicity and organ failure. Ferric citrate generally causes less irritation versus simple iron salts, a fact noted in animal studies. Chronic kidney patients absorb less iron through the gut, yet still must track intake closely to avoid iron overload. Toxicologists keep pushing for data on long-term exposure from food and water sources, aiming to re-set daily intake limits if future findings point to increased risks.
Future Prospects
Things rarely stay static in the world of iron chelates. As people keep searching for cheaper, safer, and more effective ways to address iron deficiency and phosphate management, the market for new forms and modifications of ferric citrate stays active. Researchers scan for better sources of raw materials, alternative production processes with a lower environmental footprint, and labeling systems that flag contamination fast. The path forward includes developing smart-release supplements, expanded environmental uses, and fine-tuning standards to match evolving medical knowledge. Real progress depends on transparency, ongoing research investment, and awareness of public health needs—every player in the chain from mine to medicine cabinet has a stake in getting both the chemistry and the care right.
What is Ferric Citrate used for?
What People Use Ferric Citrate For
Ferric citrate gets a lot of attention in medicine, especially among people dealing with chronic kidney disease (CKD). I’ve come across patients in the dialysis unit who know this compound well—doctors often prescribe it to help manage phosphate levels in the blood. When kidneys start struggling, they can’t filter out extra phosphate as efficiently, which can raise health risks. When too much phosphate builds up, bones suffer and heart issues become more likely. Ferric citrate helps by binding to phosphate in food, making the body flush it out instead of letting it build up.
Managing Iron Deficiency
Besides controlling phosphate, ferric citrate tackles a second problem: iron deficiency anemia. This isn’t just a small nuisance for people with CKD. Fatigue, weakness, and a foggy mind come along with low iron; I’ve watched families try to support loved ones who barely have the energy for daily tasks. Ferric citrate offers a practical solution since it provides the iron people with CKD often miss out on. Unlike intravenous iron that needs a needle and a clinic, ferric citrate comes in a pill, so folks can take it at home with their meals.
Clinical Evidence and Risks
Doctors back up ferric citrate with real results. A 2014 clinical trial in the New England Journal of Medicine showed it could lower phosphate and boost iron stores at the same time. I spoke with nephrologists who see these benefits firsthand—patients experience fewer symptoms from high phosphate, and blood tests show iron levels start to recover.
Still, no treatment is perfect. GI upset sometimes comes with the territory—nausea or constipation hits some users. There’s also a risk that too much iron can build up, especially if lab tests aren’t monitored regularly. This isn’t the type of pill anyone can pick up and take without a plan. Experienced healthcare teams keep an eye on blood counts and iron levels. Trust between patient and doctor matters a lot here.
Why Ferric Citrate Stands Out
People often ask: Why use ferric citrate over older phosphate binders like calcium acetate or sevelamer? For some, calcium-based options raise the threat of calcium overload and vascular calcification. Sevelamer brings its own side effects and never addresses low iron. Ferric citrate smooths out both issues, and insurance coverage has begun to catch up—more patients have access these days, although costs sometimes still hurt the pocket.
Key Takeaways and Possible Solutions
Chronic kidney disease patients face double trouble with high phosphate and low iron. Ferric citrate targets both in one daily prescription. Doctors still push for more research on long-term results, especially with CKD in earlier stages, and they highlight the need for education. Every patient deserves a chance to talk things over with a specialist before starting any new medicine.
Making ferric citrate affordable should remain a top priority. Insurance coverage plays a role, but hospitals and clinics need to advocate too. Pharmacies can help by checking in with folks about side effects or conflicts with other medication. Technology in lab tracking tools and follow-up systems can help. From what I’ve seen, teamwork between healthcare providers, patients, and families is the difference-maker in getting the most out of medicines like ferric citrate.
What are the side effects of Ferric Citrate?
Navigating Treatment: What to Watch For
Doctors often recommend ferric citrate for people with chronic kidney disease who need help managing phosphate levels. This iron-based medicine also provides extra iron, so blood iron numbers improve. While the treatment sounds promising, there are a few things folks pick up on pretty quickly right after starting these pills.
Common Problems: Upset Stomach and More
Anyone who has taken iron knows what happens to the gut. Ferric citrate acts in much the same way. Stomach pain, diarrhea, nausea, and constipation tend to show up early. Some people push through mild symptoms, but pain or frequent bathroom trips can interrupt anyone’s routine quickly. Data from clinical trials notes over 20% of users report these digestive complaints. It’s not dangerous most of the time, but it gets old fast.
I’ve seen a family member take ferric citrate and face days on end of stomach grumbling and frequent trips to the bathroom. Food didn’t taste right, and even drinking water sometimes felt uncomfortable. Going to work or social gatherings felt like a coin toss—would today be an okay day, or would that queasy feeling ruin plans?
Iron Overload: A Serious Concern
Ferric citrate delivers iron, which is great for anemia—but there is such a thing as getting too much. If lab tests show constantly rising ferritin or transferrin saturation, doctors may pull back on the prescription. Symptoms of iron overload creep up slowly: fatigue, pain in joints, or darker skin. People with a history of hemochromatosis or other issues storing iron should be especially careful. The National Institutes of Health offers strong guidance on tracking iron levels during treatment and stopping if numbers go too high.
Phosphate Drops: Risk for Bone and Muscle Health
Ferric citrate brings down phosphate, and that’s the point for kidney disease patients. But swinging the pendulum too far in the other direction leads to hypophosphatemia—too little phosphate in the blood. This throws off balance in muscles and bones. Weakness, bone pain, and confusion could follow. Lab tests flag this problem, but sometimes the signs aren’t obvious until bigger problems crop up. Regular blood work helps catch this early.
Other Side Effects: Uncommon but Possible
On rare occasions, rashes, itching, or swelling show up. Losing track of blood pressure or having trouble with the heart or liver—especially in folks with other health problems—calls for a doctor’s quick response. Any allergic reactions demand immediate medical help. Clinical trials suggest these serious events don’t happen often, but awareness helps families and doctors act fast if something seems off.
Managing Side Effects: Finding a Solution Together
No one wants to fear their medication. Staying in touch with your doctor, keeping blood tests up to date, and tracking how you feel day to day give the best shot at handling side effects early. Simple things like taking ferric citrate with food—or asking about timing and dosage—can make a big difference. There’s real relief in knowing what to watch for and having a plan if those symptoms roll in. Science moves ahead quickly, but it still takes listening to patients and learning from their stories to make treatment better for everyone.
How should Ferric Citrate be taken?
Understanding Ferric Citrate’s Role
Iron is essential for making red blood cells. People with chronic kidney disease often run low on iron, and some develop high levels of phosphate. Ferric citrate works by addressing both of these issues. It helps bring phosphate levels into a healthier range and gives the body extra iron at the same time. Doctors often recommend this supplement for folks who receive regular dialysis or have certain types of anemia. It’s not uncommon to feel a little confused at first—how to take it, what to avoid, and what to expect.
Taking Ferric Citrate: Steps That Matter
Your doctor or pharmacist sets the schedule. Most people take ferric citrate by mouth, usually with meals. Eating first helps reduce the chance of an upset stomach. Swallow each tablet whole. Crushing or chewing the tablet can mess with how your body uses the iron and can cause discomfort. I’ve talked with patients who noticed fewer stomach issues when they ate some bread or rice with the dose, so having a little food does more than improve comfort—it can help the supplement do its job.
Some folks ask if they can take it with coffee or tea. Studies show these drinks reduce how well your body absorbs iron. Calcium-rich foods, antacids, and certain antibiotics also get in the way. Space those out by at least an hour or two. Keep a simple routine—something like breakfast, ferric citrate, skip the dairy until mid-morning. Even as habits change, try to keep ferric citrate on the same daily schedule so your body can get used to it.
Staying Safe and Noticing Changes
Ferric citrate has a strong effect, so watch for signs your iron level is moving too high, or your phosphate level is dropping too low. Dark stools are common and usually harmless—the color comes from extra iron, not blood. Still, call your doctor if your stomach cramps up, you feel dizzy, or you notice an unusual rash. These signals mean it’s time for a checkup, not just a wait-and-see.
People with a history of iron overload or hemochromatosis need to stay cautious. Ferric citrate isn’t for those who already store too much iron. Blood tests show how treatment is going. Following lab results closely builds trust between you and your provider and keeps your health steady.
Room for Improvement: Possible Solutions
Access to regular lab tests makes a big difference. Some clinics let patients track results with digital portals. Sharing those results with a dietitian can also be useful, since food plays such a major part in managing both phosphate and iron levels. Some patients benefit from written plans or mobile reminders, helping them stay consistent even when days get hectic. With these simple steps, ferric citrate does the job it was meant to do—lifting energy, keeping bones stronger, and making tough diagnoses more manageable.
Can Ferric Citrate be taken with other medications?
Mixing Medications: What’s at Stake
Sitting in a clinic waiting room, you watch a nurse place a bottle of pills on the table. Ferric citrate—a name you might not recognize unless you or someone in your family has kidney disease. Doctors prescribe it to help manage high phosphate levels, which can put a strain on bones and the cardiovascular system. Alongside that prescription, there’s often a long list of other medications. No wonder the question about mixing ferric citrate with other pills comes up.
The Interactions Puzzle
What happens inside the gut matters. Ferric citrate binds with phosphates, so it works best in the digestive tract. The problem? It does not pick favorites. Calcium, magnesium, thyroid drugs, and even some antibiotics can lose their punch if ferric citrate keeps them from being absorbed. The risk gets real when you rely on these medicines for bone health, infection treatment, or keeping your hormones balanced.
Doctors keep an eye on this. Research out of several kidney clinics points to drugs like levothyroxine and ciprofloxacin showing lower blood levels when taken with iron-based binders like ferric citrate. The American Society of Nephrology highlights how this can lead to missing the benefits of your treatments. That means constantly watching for symptoms creeping back in, even after taking your medicines exactly as prescribed.
Personal Experience With Medication Management
Family members with chronic kidney disease have their pillboxes lined up for the week. Scheduling pills became a daily routine. If ferric citrate lands at breakfast, thyroid medicine has to wait until lunch. Putting a few hours between these doses makes a difference. This kind of planning stops one drug from blocking the other. It’s not always easy to stick to a schedule around work, meals, and family obligations. It turns into a balancing act that only gets trickier with more pills prescribed.
The Role of Healthcare Guidance
Even with careful planning, figuring things out alone does not always work. Pharmacies print warning labels, but quick chats with pharmacists or doctors offer the most help. They have access to updated databases on drug interactions. More often than not, they catch things patients might miss. Their advice shapes how people line up their medications each day. For example, some professionals suggest spacing certain drugs by at least two hours from ferric citrate. These small tweaks can mean the difference between controlling a patient’s phosphate levels and risking complications.
Potential Solutions To Keep in Mind
Digital tools now let people set reminders so different medicines don’t overlap. Some clinics use shared medication schedules that families can pull up on their phones. This keeps everyone on the same page, especially when changes happen after checkups. Keeping a written list and bringing it to every doctor visit also puts the patient in control. The more details people know about their daily schedule, the better they handle unexpected changes, like hospital stays or travel. Clear communication with healthcare providers stands out as the key step.
Managing medicines can feel overwhelming, especially as prescriptions add up. Knowing ferric citrate doesn’t play well with every drug puts the power back in patients’ hands. Real, practical steps can smooth the bumps that come with long-term treatment.
Who should not take Ferric Citrate?
Understanding Ferric Citrate and Its Place in Medicine
Ferric citrate serves as a phosphate binder and iron replacement therapy for people who often struggle with chronic kidney disease. Doctors turn to it for patients who have high phosphate levels or anemia from low iron. The idea is to manage minerals that are out of balance. Like any pill or supplement, ferric citrate comes with warnings. Not everyone should take it; serious problems can develop if ignored.
People With Iron Overload Disorders
Some folks, including those diagnosed with hemochromatosis, should stay away from ferric citrate. This disease causes the body to store too much iron. Taking more iron, in this case, only speeds up damage. Excess iron piles up in the heart, liver, and joints. Organ failure sneaks up if this buildup continues. Doctors see this in blood tests when ferritin and transferrin saturation creep too high. For anyone with a family history of iron overload, checking iron status before picking up ferric citrate proves smart.
Those With Allergy to Iron Compounds or Citrate Salts
Allergies can make a simple pill deadly. Signs show up as hives, difficulty breathing, or swelling, particularly soon after taking the first dose. If someone knows about a past reaction to iron-based products or citrate salts, they should tell a healthcare provider openly. Even one episode means steering clear of ferric citrate the next time around. Many allergists point to the body’s strong response as a reliable reason to quit the drug.
People With Advanced Liver Disease
The liver processes almost everything we swallow. When the liver fails, toxic buildup sneaks in. Ferric citrate can load more iron into a liver that’s already struggling. Patients with cirrhosis or other long-term liver issues enter dangerous territory here. Studies have linked high iron to worse outcomes in this group, speeding up complications. Doctors tend to look out for warning signs—yellowing eyes, unexplained swelling, confusion—before adding anything containing iron to a daily regimen.
Children and Pregnant People
Pediatricians almost always avoid ferric citrate in kids. Children’s bodies handle minerals differently, and there's no guarantee on safety. Side effects such as stomach pain or more serious reactions turn up faster and with less warning in younger patients. Pregnant people belong in another group where safety remains unclear. Not enough research proves ferric citrate won’t harm the baby. The FDA classifies many drugs with a strict warning for this reason. Doctors stick to better-tested options unless nothing else works.
People With Ongoing Gastrointestinal Bleeding or Ulcers
I’ve seen what happens when someone swallows iron supplements with an open ulcer or gut bleed. Stomach pain gets worse fast and stools turn black, which sometimes hides real blood loss. Ferric citrate can mask these warning signs, letting more serious problems fester. For these patients, it’s better to treat the cause of bleeding and heal the gut lining before even thinking about iron replacement like ferric citrate.
Watching for Signs and Seeking Alternatives
Everyone’s body handles iron in its own way. Routine blood monitoring paints the real picture—whether iron builds up or stays low. Trust in regular feedback from experienced providers cuts down on the risks. For those who shouldn’t take ferric citrate, other phosphate binders or ways to boost iron may work just as well. Better to ask questions, understand risks, and get the facts before adding a new drug to the mix.
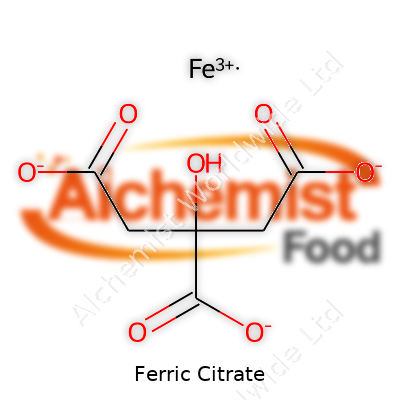
| Names | |
| Preferred IUPAC name | iron(3+) 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Iron(3+) citrate Ferric ammonium citrate Iron citrate Citrate de fer(III) Ferric tris-citrate |
| Pronunciation | /ˈfɛrɪk ˈsɪtrət/ |
| Preferred IUPAC name | iron(3+) 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Ferric Ammonium Citrate Iron(3+) citrate Iron citrate Ferric citrate hydrate Citrate of iron |
| Pronunciation | /ˈfɛrɪk ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 3522-50-7 |
| Beilstein Reference | \"Beilstein Reference 394115\ |
| ChEBI | CHEBI:31357 |
| ChEMBL | CHEMBL2108750 |
| ChemSpider | 68968 |
| DrugBank | DB12812 |
| ECHA InfoCard | ECHA InfoCard: 100.043.866 |
| EC Number | 206-619-8 |
| Gmelin Reference | 72989 |
| KEGG | C15267 |
| MeSH | D050436 |
| PubChem CID | 2954229 |
| RTECS number | GE4375000 |
| UNII | 3KX376GY7L |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID3039242 |
| CAS Number | 3522-50-7 |
| Beilstein Reference | 3568989 |
| ChEBI | CHEBI:31341 |
| ChEMBL | CHEMBL2107791 |
| ChemSpider | 69415 |
| DrugBank | DB12810 |
| ECHA InfoCard | 13cbe6aa-6c6a-40c5-8a12-5fc772e70144 |
| EC Number | 206-619-8 |
| Gmelin Reference | 68235 |
| KEGG | C18546 |
| MeSH | D050436 |
| PubChem CID | 497964 |
| RTECS number | GE7520000 |
| UNII | U88V2OFC9H |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID4086510 |
| Properties | |
| Chemical formula | C6H5FeO7 |
| Molar mass | 244.94 g/mol |
| Appearance | reddish brown powder |
| Odor | Odorless |
| Density | Density: 1.5 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -4.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.1 |
| Basicity (pKb) | 9.4 |
| Magnetic susceptibility (χ) | −98.0×10⁻⁶ cm³/mol |
| Dipole moment | 0.0 D |
| Chemical formula | C6H5FeO7 |
| Molar mass | 244.94 g/mol |
| Appearance | Reddish-brown powder |
| Odor | Odorless |
| Density | 1.5 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -2.5 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.13 |
| Basicity (pKb) | 10.7 |
| Magnetic susceptibility (χ) | −44.0×10⁻⁶ cm³/mol |
| Viscosity | Viscous liquid |
| Dipole moment | 2.78 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 203.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2174 kJ/mol |
| Std molar entropy (S⦵298) | 247.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1934.7 kJ/mol |
| Pharmacology | |
| ATC code | V03AE02 |
| ATC code | V03AE02 |
| Hazards | |
| Main hazards | May cause irritation to eyes, skin, and respiratory tract. Harmful if swallowed. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (Oral, Rat): 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50 > 2,000 mg/kg |
| NIOSH | ST425 |
| PEL (Permissible) | 15 mg/m3 (total dust) as iron |
| REL (Recommended) | 10 - 12 g/day |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | Precautionary statements: P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 oral rat 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Ferric Citrate: "1872 mg/kg (oral, rat) |
| NIOSH | WXJ305000 |
| PEL (Permissible) | 15 mg/m3 (total dust) (as Iron) |
| REL (Recommended) | 30 mg/kg |
| Related compounds | |
| Related compounds |
Ferric ammonium citrate Ferric edetate Sodium ferric gluconate complex Iron(II) citrate Ferric pyrophosphate |
| Related compounds |
Ferric sulfate Iron(III) chloride Ferrous fumarate Ferrous gluconate Ferrous sulfate |

