Ferric Chloride: Unpacking an Iron Compound’s Past, Purpose, and Prospects
Historical Development
People have been working with iron compounds for centuries, and ferric chloride, also known as iron(III) chloride, tracks back through the early experiments of alchemists. Early records from the Middle Ages show attempts to isolate chloride-based substances from mineral sources. Scientific understanding picked up in the late 18th and 19th centuries, when iron salts were systematically classified and their uses expanded. Industrial-scale production started around that time, as city waterworks and textile manufacturers looked for agents to help fight impurities and produce colorfast fabric. The story of ferric chloride is closely tied to the rise of modern chemistry: a simple solution for common, stubborn problems.
Product Overview
Walk through a water treatment plant and you’ll probably see vats of ferric chloride. It acts as a coagulant, dropping out fine particulates from water, and plays a similar role in wastewater. Printed circuit board manufacturers lean on the etching properties of ferric chloride, using it to carve precise patterns in copper. Beyond industrial sites, various grades turn up in laboratory settings and specialty chemical production. People tend to overlook just how central this compound is, but its importance stretches across both municipal infrastructure and tiny electronics labs.
Physical & Chemical Properties
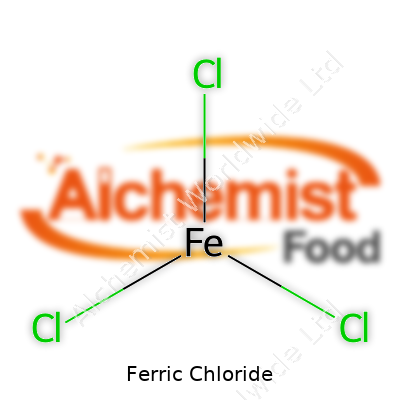
In its pure form, ferric chloride comes as black or dark brown crystals. Exposed to moisture, it dissolves in water to yield a yellowish, brown solution with a sharp, acidic odor, courtesy of hydrolysis and hydrochloric acid vapors. The compound absorbs moisture from the air, so it clumps up unless packaged well. Beyond appearance, ferric chloride is corrosive and strongly reacts with metals and organics. Its molecular formula is FeCl3, and it doesn’t take much to spot its harsh, almost metallic tang when a bottle pops open. That same reactivity drives its applications but also demands careful handling.
Technical Specifications & Labeling
Chemical suppliers provide ferric chloride in a range of concentrations and purities. Most industrial users buy 40% solution by weight, clearly marked with hazard pictograms, UN shipping numbers, and details about corrosivity and product purity. Regulations require lots to list batch numbers, expiry dates, and manufacturer details, partly to ensure traceability if something goes wrong at a treatment plant or etching facility. Standard labeling usually calls for compliance with GHS, OSHA, and sometimes REACH, depending on the region of sale. Packaging varies: drums for small customers, ISO tanks for bulk handlers, each one tested for compatibility and leak-proofing.
Preparation Method
Most commercial ferric chloride comes from direct chlorination of scrap iron or iron ore. Typically, technicians feed excess dry chlorine gas over hot iron in a sealed reactor. The reaction cranks out pure FeCl3 vapor, which condenses out as a granular solid or gets dissolved immediately for liquid delivery. Other manufacturers rely on dissolving iron oxides or hydroxides in hydrochloric acid, using heat to drive off water and unwanted gases. Choice of production method depends on feedstock availability, scale, and local regulations about emissions or byproducts. Each step in the chain leaves fingerprints in the trace contaminants, so reputable suppliers invest in process control and post-reaction purification to keep their claims solid.
Chemical Reactions & Modifications
Ferric chloride comes alive through its strong oxidizing potential. Drop it into water, and FeCl3 hydrolyzes, forming iron(III) hydroxide and releasing acidic protons. Industrial chemists often use it in Fenton-like reactions or as a mild chlorinating agent. It picks up electrons from organic substrates in etching, stripping away metals and converting them to soluble metal chlorides. Ferric chloride’s reactivity allows modification into double salts, like potassium ferric chloride, for finer tuning in specific applications. Its role in Friedel-Crafts reactions (as a Lewis acid catalyst) has made it a workhorse in organic synthesis, offering pathways into dyes, pharmaceuticals, and specialty polymers.
Synonyms & Product Names
Markets and chemists call ferric chloride by several names, including iron(III) chloride, ferric trichloride, and even dechlorane at times. Product listings sometimes use variations in spelling and punctuation depending on country or end use. In older chemical supply catalogs, one might spot “perchloride of iron,” although this term has faded with standardization efforts. CAS number 7705-08-0 is the global identifier, but shipment documents may also flag EINECS or UN designators. PCB manufacturers and water plant operators know it as “etchant” or “coagulant,” signaling its main function on-site.
Safety & Operational Standards
People handling ferric chloride deal with serious hazards. The compound attacks skin and eyes on contact, and inhalation of dust or fumes can burn lungs. Direct splashes eat through fabric and corrode metal fixtures. Companies rigorously train workers to use goggles, acid-resistant gloves, and full aprons. Emergency wash stations line the walls of most loading docks, not as window-dressing, but as a last defense against slip-ups. OSHA, NIOSH, and local authorities publish clear guidelines for storage: dry, secure, and away from incompatible materials. Routine checks ensure tanks, pumps, and valves resist corrosion, with regular testing for leaks. Transport falls under hazardous materials regulations, calling for honest paperwork and real-time spill response plans.
Application Area
Ferric chloride makes its biggest mark in water treatment. Flocculation tanks in municipal plants churn with brownish solution, as it grabs fine particles and helps remove contaminants that settle out for easier filtering. Printed circuit board factories run hundreds of gallons through etching baths, transforming unremarkable copper sheets into smartphone brains. Textile and dye industries lean on ferric chloride for oxidation processes, sustaining color and fastness in synthetic fabrics. In the lab, researchers tweak its concentration to precipitate proteins or to trigger redox reactions in organic syntheses. Some niche uses include pigment production for paints and as a mordant for historic photographic printing techniques. Day-to-day life owes a quieter debt to this sharply reactive salt.
Research & Development
Research labs keep looking for new tricks with ferric chloride, hoping to lower environmental impact or boost performance. Scientists investigate membrane-friendly modifications, where the salt acts as a cross-linker in advanced filtration systems. Some explore fine-tuning etching processes for microfabrication, aiming for sharper lines or less waste by using ferric chloride with additives and surfactants. Water treatment technologists examine hybrid coagulant blends, leveraging ferric chloride with organic polymers to snag contaminants conventional methods let slip through. Analytical chemists also push the envelope by developing sensors and colorimetric assays based on ferric ion’s distinct reactivity. Continuous scaling and process intensification become focal points, trimming costs and tightening purity windows.
Toxicity Research
Lab results and field observations agree: ferric chloride, while less toxic than many heavy metal salts, poses health risks if mismanaged. Direct exposure leads to chemical burns, and studies in rodents flag gastrointestinal damage with high oral intake. Environmental scientists track runoff from water plants and industrial users, noting that iron and chloride ions can stress aquatic ecosystems, especially if discharged in excess. Much research focuses on understanding pathways for uptake in humans and animals: how much makes it into the bloodstream, what metabolic routes process it, and where iron overload risks emerge. Regulatory agencies regularly review workplace exposure limits, and ongoing research supports safe disposal practices, closed-loop recovery, and monitoring downstream effects.
Future Prospects
With water scarcity and electronics manufacturing on the rise, ferric chloride’s star won’t fade any time soon. Researchers experiment with next-generation coagulants—based on greener, biodegradable alternatives—but so far, few match ferric chloride’s balance of cost and effect. Innovations in recovery and recycling, including on-site regeneration systems, show promise for reducing waste and curbing costs in big municipal plants. Meanwhile, as technology presses for finer traces on circuit boards, formulation experts keep optimizing additives and bath temperatures to keep production humming with minimal scrap. Environmental concerns shape ongoing development, with companies trialing low-impact handling, sealed systems, and real-time monitoring to prevent accidental releases. Ferric chloride remains both an unsung backbone of industry and a candidate for smarter, cleaner solutions as pressures mount for sustainability.
What is Ferric Chloride used for?
From Rusty Orange to Critical Use
Most people walk by rusty stains on metal and never think twice. But the compound behind a lot of that color—ferric chloride—keeps surprising me every time I come across it in wastewater plants or photos of printed circuit boards. Ferric chloride’s not just another chemical sitting in a list. This stuff means clean water, working electronics, and a reminder that even humble chemistry can end up shaping our daily lives.
Keeping Water Drinkable
For anyone who values a clean glass of tap water, ferric chloride plays a starring role you probably never noticed. Cities add it to wastewater to help pull out nasty stuff like phosphates and suspended bits, so water flowing back to rivers or into our homes isn’t loaded with contaminants. Having worked near a treatment plant, I’ve seen the brown sludge tanks after ferric chloride gets added. It clumps up fine particles, dragging them down so they can be filtered out. Chlorine-based chemicals sometimes get a bad rap, but ferric chloride passes a lot of scrutiny from health experts because it’s effective at the doses used. The process helps keep algae blooms at bay and helps towns keep their environmental footprint smaller.
Electronics and Etching
Every circuit board in your phone or laptop likely owes something to ferric chloride. In high school, I etched copper traces onto DIY boards with it, carefully pouring the deep orange liquid over a design drawn with a resist marker. The ferric chloride chews away the exposed copper, revealing the desired pattern. This same technique fills manufacturing plants worldwide, powering a multi-billion-dollar electronics industry. Sure, fancier methods now whiz by with lasers and advanced resists, but ferric chloride still handles much of the dirty work for quick prototypes or smaller production runs. Its low cost, reliability, and ready availability keep it relevant, even as gadget manufacturing moves faster than ever.
Steel, Stains, and Surprising Art
Ferric chloride’s tough reputation also earned it a spot with steelmakers. It tests metals to check for flaws, especially on tools and blades that need to stay sharp and resistant to breaking. In some shops, artists use ferric chloride to develop dramatic surface patterns on metal objects, including knives and jewelry. The chemical brings out subtle details and textures, giving craftspeople a dependable and creative tool. I’ve watched a knife maker dip a blade in a diluted solution, revealing patterns that looked almost like topography maps—no two blades alike.
Risks Aren’t Just Lab Tales
While ferric chloride belongs in many industries, the people working with it can tell you it needs respect. It is corrosive and stains clothes, hands, and concrete almost instantly. Safety training and good equipment matter every day in treatment plants and workshops. Local governments don’t let it leak into sewers without neutralization because it’s toxic to aquatic life at high concentrations. Chemical spills from factories have stirred up concern, especially in developing countries lacking strong waste controls. For me, seeing a splash on a workshop floor made it clear how easy mistakes can happen without clear-eyed handling and rules.
Working Toward Safer and Smarter Solutions
Many companies now look for ways to cut down on loss and waste. Double-walled tanks, leak alarms, and employee education reduce the odds of ferric chloride escapes. Cleaner water and safer electronics don’t call for shortcuts—they call for responsibility. More cities now share their chemical handling methods with the public, building trust with the people they serve. Investing in research on recovery and recycling of chemical residues from etching could close some loops, saving both money and the environment. Every improvement becomes another step away from preventable accidents and toward a culture that values skill as much in storage and disposal as we do in all the creative things we use ferric chloride to accomplish.
Is Ferric Chloride hazardous or toxic?
Seeing Ferric Chloride at Work
Walk into any water treatment plant, electronics workshop, or even high school science lab, and ferric chloride probably waits on a shelf. It shows up as a brownish-yellow solid or a very dark solution, and people use it to etch circuit boards, purify drinking water, and handle sewage. Curiosity about its safety isn’t just for chemists or engineers—anyone who grabs that bottle or works in those rooms should know: ferric chloride brings real risks to the table.
What My Experience Says
I’ve handled ferric chloride as both a chemistry student and a hobbyist who likes making printed circuit boards by hand. I learned the hard way that this isn’t a substance you treat lightly. Skin burns and yellow stains stick around for days if you aren’t careful. A splash near your eyes calls for a quick emergency rinse or a possible trip to the doctor. Breathing in its dust or mist feels harsh, too—it burns in your nose and throat, leaving you coughing and feeling off for hours.
Medical and Scientific Evidence
Ferric chloride acts as a strong oxidizer and reacts powerfully with moisture—especially skin or eyes. According to occupational safety organizations, ferric chloride can cause serious irritation or damage where it touches tissue. Accidental ingestion ends up with nausea, abdominal pain, or even more serious medical emergencies, like liver or kidney toxicity, depending on how much gets into the system.
The Material Safety Data Sheets back up that personal experience. They list warnings for corrosivity, respiratory hazards, and environmental risks if you pour large amounts down a drain. Researchers have even pointed out risks to aquatic life: water contaminated with ferric chloride disrupts the delicate balance for fish and other organisms.
Why People Should Care
It’s easy to ignore the label on a bottle collecting dust. But ferric chloride has a way of catching up with anyone who works with it without gloves or goggles. Burns, caustic reactions, and environmental spills can become costly problems, whether that’s a ruined project or an expensive cleanup. When safety is skipped, the price adds up fast—in injuries, lost time, or fines from regulators who take spills seriously.
Kids or students, drawn in by curiosity or a teacher’s demonstration, face even greater risks. They might forget to mention a small splash or rub their eyes at the wrong moment. Stories spread fast about science fair projects gone wrong or chemistry clubs with unexpected trips to the nurse’s office because the safety gear stayed in a drawer.
Steps That Actually Keep People Safe
Goggles and chemical-resistant gloves aren’t optional—they’re a basic part of working with ferric chloride safely. Keeping good ventilation cuts down on breathing in any fumes or dust. In school settings, teachers should show every class how to respond to spills or splashes before the bottle even opens. At home, clear labeling, safe storage away from kids or pets, and not pouring any leftover solution into the garden or storm drain prevents accidents and fines.
For larger industries, training new employees on the risks and keeping emergency showers and eye wash stations nearby goes just as far as any high-end safety protocol. Tracking usage and taking waste to the right disposal facility matters for the health of the whole community. Ferric chloride doesn’t belong in local rivers or on bare skin—awareness, preparation, and quick action always make the difference.
How should Ferric Chloride be stored?
What Ferric Chloride Demands from Storage
Ferric chloride feels like one of those chemicals that people hear about in passing but never take the time to respect. Through work in water treatment facilities and college chemistry labs, I've seen the substance eat through concrete floors when someone overlooked simple storage rules. It’s a corrosive, moisture-loving compound, and leaving it in the wrong place is just asking for trouble.
Strong oxidizers like ferric chloride react pretty much instantly with many common materials, especially metals. Pouring it into a steel drum won’t end well; it corrodes, leaks, and then creeps into ground or air. Glass, certain plastics (like polyethylene or polypropylene), and corrosion-resistant rubber lining hold strong. Companies selling ferric chloride usually ship it in thick, dark containers to shield it from sunlight and accidental bumps—something folks at home or in smaller outfits often ignore, thinking taping the lid shut will do.
Why Humidity and Temperature Matter
Open a container of ferric chloride on a muggy day, and you’ll see it attract moisture like a sponge in a rainstorm. What people don’t realize is, this isn’t just about keeping the solution concentrated for industrial uses—moisture changes the chemical, produces hydrochloric acid fumes, and makes the liquid gnaw through nearly everything nearby. Keeping storage rooms dry, even if it means spending a little more on dehumidifiers or ventilation, pays off.
Temperature swings cause pressure changes inside containers, which can buckle weak seals or make vapors escape. I’ve watched makeshift lids blow off in summer heat. Just as with common household bleach, ferric chloride tanks belong away from direct sun or heat sources, in well-ventilated rooms that rarely climb above standard room temperature. Not everyone has a climate-controlled warehouse, but at the very least, a shaded, cool, and locked cabinet away from other chemicals does the trick.
Health and Environmental Stakes
Ferric chloride’s immediate risks can seem overblown—until a splash lands on skin or eyes. Burns and lasting stains happen in seconds. Even more worrying: small leaks build up, and fumes drift. Inhaled over time, even at low levels, ferric chloride stirs up respiratory trouble. Most chemical accidents happen when people downplay these risks. Proper storage isn’t just about preventing “big” emergencies— it’s about not slowly poisoning the workspace or local water supply slot by slot.
Runoff from spilled ferric chloride spills into drains or soil, and water authorities face a cleanup nightmare. The US Environmental Protection Agency places strict rules on ferric chloride disposal for a good reason. In regions where drinking water sources already struggle with heavy metals, dumping or leaking from careless storage aggravates long-term health effects. From a professional standpoint, putting safety data sheets on the wall does less good than actually reading them—and making storage safety a routine, not a yearly check-in.
Spotting and Fixing Weak Links
Many problems show up at container seams, old valves, or neglected secondary containment trays. Small cracks or brittle gaskets spell out disaster if products sit for months. Regular checks and swapping old parts don’t cost much—certainly less than a hazmat team mopping up. Color-coded bins, spill kits, and real training pay off more than any lock or warning sign.
Ferric chloride shouldn’t scare people away from essential water or electronics work. Planned right, storage means thinking ahead and fearing laziness more than the chemical. Walking through storerooms, checking for cool, dry spaces, and staying alert to corrosion keep the community safe and business running without headlines the next day.
What are the safety precautions when handling Ferric Chloride?
Handling a Tough Chemical
Ferric chloride gets a lot of use in water treatment plants and electronics workshops. It etches copper off circuit boards, cleans industrial waste, and even manages odor in sewage. The stuff makes life easier on the job, but nobody wants it on their skin or in their lungs. One wrong move and a good day turns into the wrong kind of story.
Why Respect Is Non-Negotiable
I’ve seen firsthand what ignoring safety gear leads to. Red splotches on hands, the sting that doesn’t go away, and once, a coworker’s ruined glasses because a drop splashed up. Ferric chloride doesn’t mess around. Its acidity eats through organic material quickly, corroding metal, too. Breath in its fumes enough, you’ll feel it. Eyes and lungs start burning fast. According to the CDC, contact with skin or eyes can cause severe irritation and damage, and inhaling the dust or mist does a number on respiratory health. OSHA warns about chronic exposure leading to persistent coughing or asthma-like symptoms.
Gear Up, Don’t Skip Corners
The right protection matters more than speed or convenience. Chemical resistant gloves—nitrile or neoprene—keep the liquid out. Lab coats or long sleeves block splashes from bare arms. Goggles with side shields beat regular safety glasses every time, and a face shield gives peace of mind when pouring or mixing. Add a proper mask or respirator if you’re not working in a well-ventilated area. Most shops rely on a sturdy apron and nonslip shoes. I’ve scuffed mine more times than I can count, but they keep the toes out of trouble when spills happen.
Keep the Workspace in Check
Good airflow makes a world of difference. Fume hoods help a lot if you have them, and open windows or fans work for smaller setups. Bottles belong on sturdy benches away from walkways, marked with clear hazard labels. Don’t trust memory—always double-check containers before pouring. Dedicated storage for acids, far from anything flammable or reactive, means emergencies don’t become disasters. I always keep baking soda handy; it neutralizes spills fast. A spill kit close by, not stuck in a back room, is an easy win.
Smart Habits and Training
No one plans on accidents, so practice goes a long way. Take five minutes to read labels again before each use. If something doesn’t smell right, stop and get help. Rinsing a splash with water beats toughing it out. Show new folks where the eyewash station and shower are before they ever open a bottle. Safety data sheets (SDS) don’t gather dust—they’re a tool, not a formality. I keep copies close wherever this chemical turns up.
Managing Disposal
Used ferric chloride needs careful attention. Pouring leftovers down the sink causes real trouble for pipes and the environment. Local laws tell you where to take old solution, and hazardous waste facilities handle what’s too risky for trash. I always double-seal waste in chemical-proof containers and log every drop in a disposal sheet. Cleaning up the right way keeps regulators happy and the workplace safe for everyone who comes next.
Simple respect and a few extra steps turn ferric chloride into a useful tool instead of a hazard. Extra ten seconds with gloves or a check around the workspace feels minor until the day it keeps you out of the emergency room.How is Ferric Chloride disposed of after use?
Understanding Ferric Chloride’s Journey After Use
Ferric chloride shows up in lots of real-world jobs: treating water, etching circuit boards, and cleaning up industrial waste. Once it’s done its job, though, you’re left with a substance that’s no friend to human health or the environment. At work in a water plant, I learned how dangerous even a spill can be. Skin burns, toxic fumes, dead fish in the river—these risks are not abstract. They’re real, and they turn a routine day into a crisis.
Wastewater Treatment: Not a Dump Zone
Some folks imagine you can pour ferric chloride down the drain and let the water plant handle the rest. The truth looks messier. Ferric chloride in solution is acidic and reactive. Even a small leak corrodes metal, eats through concrete, and releases chlorine-based gases. Pouring it away piles the burden onto already-overtaxed municipal systems and threatens bacteria populations in treatment tanks—the same ones that break down waste for all our cities. Studies from the EPA show that pH swings from chemical dumping cause fish die-offs and algae blooms downstream. Once the ecosystem tips out of balance, it takes years to bounce back.
Neutralization: A Hands-On Approach
Some manufacturers and labs tackle ferric chloride sludge by neutralizing it on-site with lime or sodium hydroxide. It’s not complicated on paper. Add base slowly, track the pH, and aim for that sweet spot near 7. This process produces iron hydroxide, a solid that separates out. In my own lab work, I saw failures pop up just from trying to rush: adding too much quicklime made violent reactions and clouded the air with steam and dust. Proper neutralization takes patience, good ventilation, and simple but sturdy gear—plastic buckets, thick gloves, goggles, pH paper. The goal isn’t just compliance with a regulation: it’s protecting the people on the shop floor and everyone who drinks downstream water.
Landfilling the Byproducts—Not a Free Pass
Once you’ve got that sludge, the next question comes up fast: where does it go? Dumping it into municipal landfills comes with a long string of permits and rules. Landfills don’t take everything. Iron hydroxide from this process sometimes falls under hazardous waste rules, especially if it carries heavy metal contamination from a plating bath or electronics work. Waste must travel in sealed containers, with tracking paperwork for every step, or else leaks edge their way into the soil. The EPA’s Toxic Release Inventory lays out how poorly managed ferric chloride byproducts end up in water tables, causing iron overload in plants, stunted fields, and even stained drinking water.
Finding Better Paths Forward
Some companies look beyond treatment and landfill. Iron hydroxide sludge sees new life in brick manufacturing or as pigment in concrete and ceramics. This isn’t wishful thinking—it’s already happening in plants across Europe. These options cut down landfill use and keep hazardous waste from sitting around for generations. Real progress takes input from regulators, workers, and communities, all pulling in the same direction. I’ve seen how small tweaks—like dedicated spill trays, better training, and quicker containment of leaks—shift the odds away from disaster and toward something safer and cheaper.
Why It Matters
Living with ferric chloride’s leftovers demands more than a checklist or a quick rinse. Protecting water, soil, and workers takes active management and open eyes for new ideas. Everyone shares a stake—whether you’re running a treatment plant, checking fish in the river, or turning on the tap at home.
| Names | |
| Preferred IUPAC name | iron(III) chloride |
| Other names |
Iron(III) chloride Ferric trichloride Iron perchloride Iron chloride FeCl3 |
| Pronunciation | /ˈfɛrɪk ˈklɔːraɪd/ |
| Preferred IUPAC name | Iron(III) chloride |
| Other names |
Iron(III) chloride Ferric trichloride Iron sesquichloride Iron chloride FeCl3 |
| Pronunciation | /ˈfɛrɪk klaɪˈraɪd/ |
| Identifiers | |
| CAS Number | 7705-08-0 |
| Beilstein Reference | 14022 |
| ChEBI | CHEBI:30812 |
| ChEMBL | CHEMBL1201131 |
| ChemSpider | 21520 |
| DrugBank | DB09114 |
| ECHA InfoCard | 100.097.839 |
| EC Number | 231-729-4 |
| Gmelin Reference | 970 |
| KEGG | C18798 |
| MeSH | D004487 |
| PubChem CID | 24380 |
| RTECS number | AGQ70000E |
| UNII | E1H42KYXOT |
| UN number | UN2582 |
| CAS Number | 7705-08-0 |
| Beilstein Reference | 358781 |
| ChEBI | CHEBI:30812 |
| ChEMBL | CHEMBL1201480 |
| ChemSpider | 21519 |
| DrugBank | DB09225 |
| ECHA InfoCard | 100.097.812 |
| EC Number | 231-729-4 |
| Gmelin Reference | Gmelin Reference: 14008 |
| KEGG | C00439 |
| MeSH | D005247 |
| PubChem CID | 24380 |
| RTECS number | BR9050000 |
| UNII | E1J6Z7B94R |
| UN number | UN 2582 |
| Properties | |
| Chemical formula | FeCl3 |
| Molar mass | 162.20 g/mol |
| Appearance | Dark brownish-black solid or liquid |
| Odor | Chlorine-like |
| Density | 2.9 g/cm³ |
| Solubility in water | 744 g/L (20 °C) |
| log P | -4.0 |
| Vapor pressure | 1 mm Hg (20°C) |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | -8 |
| Magnetic susceptibility (χ) | +1230·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.56 |
| Viscosity | Low viscosity |
| Dipole moment | 0.00 D |
| Chemical formula | FeCl3 |
| Molar mass | 162.20 g/mol |
| Appearance | Dark brown crystalline solid or solution |
| Odor | Chlorine-like |
| Density | 2.8 g/cm³ |
| Solubility in water | 740 g/L (20 °C) |
| log P | -4.39 |
| Vapor pressure | 1 mmHg (20°C) |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | -8.0 |
| Magnetic susceptibility (χ) | +522.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.615 |
| Viscosity | Low viscosity |
| Dipole moment | 0.00 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 242.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -399.5 kJ/mol |
| Std molar entropy (S⦵298) | 199.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -399.5 kJ/mol |
| Pharmacology | |
| ATC code | B03AB01 |
| ATC code | B03AB01 |
| Hazards | |
| Main hazards | Corrosive, causes burns to skin and eyes, harmful if swallowed, inhalation may cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H290, H314 |
| Precautionary statements | P234, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P363, P405, P501 |
| NFPA 704 (fire diamond) | 3-0-1-acid |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 (oral, rat): 900 mg/kg |
| LD50 (median dose) | 450 mg/kg (Rat, oral) |
| NIOSH | DJ8925000 |
| PEL (Permissible) | PEL: 1 mg/m³ |
| REL (Recommended) | 2 mg/m³ |
| IDLH (Immediate danger) | 100 mg/m3 |
| Main hazards | Corrosive, causes burns to skin and eyes, harmful if swallowed or inhaled, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H290, H314, H302 |
| Precautionary statements | P234, P260, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P363, P405, P501 |
| NFPA 704 (fire diamond) | 3-0-1-Acidos |
| Lethal dose or concentration | LD50 (oral, rat): 900 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 900 mg/kg |
| NIOSH | VL8575000 |
| PEL (Permissible) | PEL: 1 mg/m³ |
| REL (Recommended) | 1 mg/m³ |
| IDLH (Immediate danger) | 100 mg/m3 |
| Related compounds | |
| Related compounds |
Iron(II) chloride Iron(III) sulfate Iron(III) nitrate Aluminium chloride Copper(II) chloride |
| Related compounds |
Iron(II) chloride Aluminum chloride Copper(II) chloride Ferric sulfate Ferrous sulfate Iron(III) nitrate |

