Ferric Ammonium Citrate: Substance with a Past, Present, and Future
Historical Development
Ferric ammonium citrate turns up in textbooks long before antibiotics and modern chemistry labs filled pharmacy shelves. Back in the mid-19th century, iron compounds drew attention for both nutrition and industry. This complex found a place on the chemist’s bench thanks to its unique solubility and versatility. I remember scanning old chemical supply catalogs and seeing ferric ammonium citrate among the stained glass pigments and cyanotype chemicals—names that echo through science history. In Victorian hospitals, doctors turned to these salts in their battle against anemia, while scientists found other uses in early photography and blueprint production. These roots matter today, showing just how deeply this compound plugs into everyday science and health.
Product Overview
Ferric ammonium citrate comes as a pale green or brown powder, easy to spot in a supply room. This material combines ferric ions, ammonium, and citrate into a loosely structured salt. Water dissolves it in a snap, which beats working with more stubborn iron compounds. Pharmaceutical companies—looking for both iron source and chemical stabilizer—keep ordering it. In food processing and imaging, its mild taste and lack of strong odor help it pass muster. Holding together in so many fields, ferric ammonium citrate shows both flexibility and performance in real-world settings.
Physical & Chemical Properties
The powder tells much about its story. Its color ranges from light green to reddish brown, depending on how it’s made. Water dissolves it easily, but alcohol and ether do not; there’s no strong scent, and it clumps when humidity rises. This compound won’t burn but starts to break up if temperatures go past 200°C, shedding ammonia and shifting color. Chemically, it is a mix: ferric ions wrapped with citrate groups and balanced with ammonium, yielding high solubility and a complex ability to react with light and metal ions. Its oxidizing nature leads to gentle reactivity with reducing agents.
Technical Specifications & Labeling
Manufacturers post tight specs: iron content falls between 16% and 18%, while ammonium clocks in at about 7%—checked by regular titration and colorimetry. Bulk density lands between 0.6 and 0.9 g/cm³, and loss on drying stays under 12%. Labels must show batch number, expiration date, and concentrations of possible residual metals, including lead and arsenic. Global regulators demand detailed safety warnings, and—because ferric ammonium citrate sometimes enters food or supplement channels—allergen and country-of-origin information stays front-and-center.
Preparation Method
Labs and factories usually whip up ferric ammonium citrate by mixing ferric chloride with citric acid in water, then adding ammonia to raise the pH. Constant stirring and temperature control keep things predictable. After full reaction, the solution shows the classic green or brown color. Removing water—most commonly through spray drying—leaves the finished powder. Impurity checks, especially for unreacted iron salts, finish the process.
Chemical Reactions & Modifications
Under light, ferric ammonium citrate breaks down, freeing up iron; this property gave birth to blueprint and photographic printing. Adding reducing agents, like ascorbic acid, unlocks ferrous iron. Acids shift its composition, and strong bases strip off ammonium. Chemists sometimes tweak ratios for specific reactivity, producing heavier or lighter iron loads or stabilizing with extra ammonium to buffer against pH swings. Modern research has nudged this compound into new roles—such as nanostructure synthesis—with minor modifications showing big effects.
Synonyms & Product Names
Different catalogs and countries refer to it in different ways: ammonium ferric citrate, iron(III) ammonium citrate, and, on pharmacy shelves, it may just appear as “ammonium iron citrate.” Sometimes color designates grade—“green” or “brown”—reflecting iron and citrate content. Medical supplements and technical-grade batches carry separate labels, but the root compound stays the same.
Safety & Operational Standards
Safety matters more than ever, especially with a substance that can cross into both medical and industrial channels. Iron compounds call for careful handling; low-level chronic exposure in workspaces brings health risks—mainly respiratory irritation and, in rare cases, iron overload. GHS guidelines rank ferric ammonium citrate as slightly hazardous, and proper protective gear—gloves, goggles, and dust masks—belongs in every protocol. Workplace ventilation keeps dust away from lungs. Spills clean up with water and neutral detergent; strong oxidizers and combustibles stay well away. Lab supervisors and health professionals rely on regular training, safety audits, and up-to-date MSDS documentation to keep risks in check.
Application Area
This compound travels through many industries. Supplement makers use it for iron fortification, since gastrointestinal absorption runs higher than that of standard ferrous salts. In cyanotype imaging—the process behind classic blueprints—its photo-reactive iron lies at the core of the method. Water treatment plants add it to bind arsenic and other heavy metals for easier removal. Cheese factories leverage its stabilizing action as an additive. Research labs test its properties in nanoparticle synthesis and as a catalyst base. These fields share a surprising connection: all draw on ferric ammonium citrate’s high solubility and mild reactivity, not on some esoteric effect.
Research & Development
R&D teams poke at the edges of what ferric ammonium citrate can do. Nutritional scientists explore micronutrient blends with lower side effects. Green chemistry uses it as a template for iron-based catalysts in breaking down pollutants. Medical researchers test its role in imaging contrast, delivery of drugs, and wound healing. One study found improved antimicrobial coatings for medical devices by incorporating it with silver. The easy reactivity also attracts teams designing bioinspired materials—especially for slow-release iron sources or as a scaffold for nanoscale assembly. Each advance builds on decades of simple, reliable chemistry.
Toxicity Research
Toxicologists keep a close eye on iron compounds. In moderate doses, ferric ammonium citrate generally passes through the gut safely, but children’s accidental ingestion creates medical emergencies. Too much iron taxes the liver, causes oxidative stress, and, at worst, damages organs. Regulatory limits for food and supplement use reflect these risks. Animal studies confirm a margin of safety when absorbed in regulated amounts. Chronic workplace exposure appears low-risk, though regular monitoring helps. Wastewater disposal gets oversight to prevent excess iron from contaminating water sources.
Future Prospects
Looking ahead, ferric ammonium citrate won’t draw headlines, but its value rises as researchers chase safer supplements and more sustainable chemical processes. Emerging work on environmental remediation—especially water purification and soil decontamination—leans on cheap, easily-handled iron compounds. Additive manufacturing finds roles for iron complexes as redox-active ingredients in novel printing methods. As healthcare shifts toward gentler, food-based supplements, non-constipating forms of iron will keep this material under consideration. Industry trends in green chemistry, waste reduction, and advanced imaging pull ferric ammonium citrate into new experiments, keeping its future secure in both the lab and the marketplace.
What is Ferric Ammonium Citrate used for?
Mending Iron Deficiency
Iron matters to the body. It helps cells bring oxygen where it’s needed, keeps us energetic, and prevents anemia. Doctors often find themselves suggesting supplements to those who don’t get enough iron from their meals, whether it’s because of health reasons, pregnancy, or a limited diet. Ferric ammonium citrate stands out in this area because it can be absorbed easily. Pharmaceutical companies make syrups, tablets, or drops from it. These become lifelines for people with iron deficiency anemia. It’s gentle on the stomach compared to old-school iron salts, so it often gets top marks from those who otherwise find supplements tough on their digestion.
A Helper in Food Processing
Most don’t think about what goes into processed foods behind the scenes. Yet, ferric ammonium citrate gets mixed into some breakfast cereals and baby formulas. The point isn’t just iron enrichment, but to keep the final product looking and tasting right. Some iron additives can give off strange flavors or turn food funny colors. Ferric ammonium citrate steps around those problems, adding iron without weird aftertastes or color shifts. This keeps both food manufacturers and fussy kids happy.
Photography and Science Education
Cyanotype prints have a distinct blue look — a favorite among art students and photographers who like the tactile magic of historical processes. Ferric ammonium citrate handles a key job in making those images appear during cyanotype printing. Once exposed to light and washed, the bright blue designs come to life. Beyond art, teachers use this method in classrooms to spark curiosity about chemistry, light, and creativity.
Water Treatment and Environmental Uses
Clean water takes work. Small utilities and even some clinics treat their water with ferric ammonium citrate to pull out unwanted stuff. It reacts with dissolved metals or contaminants, making them clump up so they can be filtered more easily. Clean water means fewer sick days and better health for communities. This compound also cuts down waste in certain paper-making or textile industries, tying up heavy metals that would otherwise pollute waterways. Simple chemistry ends up protecting drinking water and rivers alike.
Medical Imaging and Research
Hospitals sometimes rely on ferric ammonium citrate as a contrast agent during scans of the digestive tract. X-rays spot the iron clearly, letting doctors find strictures, leaks, or tumors more easily. This improves speed and confidence when making decisions about surgery or further tests. On the research side, scientists explore its properties to design new imaging methods or study how iron moves through the body, which could give better ways to diagnose and treat disease.
Challenges and Responsibly Moving Forward
No chemical stands above scrutiny. The World Health Organization recognizes ferric ammonium citrate as safe in the right amounts, though some folks get upset stomachs if they take too much. Food makers, doctors, and regulators need to watch those limits. Clear labeling and ongoing research can stop problems before they start. More importantly, companies can partner with healthcare workers to make sure people with special dietary needs get guidance on which iron supplements fit their bodies best.
Tapping Its Goodness
From relieving tiredness to keeping food healthy, ferric ammonium citrate keeps showing up where it matters. It’s not flashy and it rarely gets much credit, but that’s exactly what makes it a vital player both in the lab and on the supermarket shelf. By respecting the science, tracking how we use it, and learning from experience, we can make the most of this compound’s benefits without running into trouble down the line.
What are the side effects of Ferric Ammonium Citrate?
Understanding the Ripple Effects
Ferric Ammonium Citrate often pops up in iron supplements, lab reagents, and sometimes in foods. If you ever dealt with low iron, you might have seen it printed on a pill bottle. The whole point is to get enough iron into the body, which helps make red blood cells and keeps energy levels from hitting the floor. But as with anything that messes with your internal chemistry, it pays to know what you’re really signing up for.
Common Problems That Crop Up
Nobody wakes up hoping for digestive upsets, but that’s one of the biggest complaints with this stuff. Stomach pain, nausea, and even vomiting can tag along after a dose. Iron supplements, no matter the form, have a reputation for causing constipation and sometimes the opposite—diarrhea. The color of your stools might change, too, often turning them black or dark green. For anyone new to iron pills, that alone can startle you into thinking it’s something way worse.
From what I’ve seen in medical practice, these side effects push people to skip doses or quit altogether, even if they know they need the iron. The irony is harsh because untreated iron deficiency causes way bigger problems, but side effects can feel like a wall you don’t want to cross.
Lesser-Known Worries
Rarer reactions deserve attention, too. Ferric Ammonium Citrate, although not the most common culprit, can trigger allergic reactions. Rashes, itching, swelling in the face or throat can show up. Trouble breathing is a big red flag and should always mean heading straight to the emergency room.
Too much iron builds up over time, and this can poison your liver, heart, and pancreas. Symptoms sneak up—joint pain, tiredness, a bronze tint to the skin, irregular heartbeats. Awareness matters when you look at the risks, especially in kids. Iron overdose stands as a leading cause of fatal poisoning in children under six who accidentally swallow iron tablets.
Why the Risk Matters in Real Life
Some people shrug off mild nausea or a change in stool color, but ongoing constipation can lead to more doctor visits, especially for older adults who already struggle with gut issues. Taking this supplement with food can soften some stomach problems but also cuts down how much iron gets absorbed. That’s a real catch-22. For me, the tradeoff between correcting iron deficiency and dealing with a queasy stomach was always a balancing act; mixing it with an orange juice at breakfast worked better than taking it straight.
Not everyone should reach for iron on their own. Conditions like hemochromatosis, thalassemia, or certain anemias mean that extra iron does more harm than good. Every bottle should carry a warning that blood work and doctor’s input aren’t optional. Too many folks buy iron supplements off the shelf, not knowing if they really need them.
Better Ways to Handle the Issue
Simple advice—start with a small dose and work upward if you tolerate it, and choose food sources of iron where possible. Lentils, spinach, red meat, and fortified cereals give plenty of iron without as much risk. Vitamin C (think oranges or peppers) helps the body take in non-heme iron from plants.
Brands now offer slow-release versions or pair iron with compounds that ease digestion, which can make all the difference. Regular blood tests help keep tabs on iron levels, and open conversations with healthcare providers let folks choose what works for their bodies and lifestyles.
Is Ferric Ammonium Citrate safe for children?
Looking Closer at a Common Iron Supplement
Most parents want healthy kids, and making sure they get enough iron is part of that. Doctors often prescribe iron supplements to treat anemia, a problem that crops up often in growing kids and teens. Ferric ammonium citrate shows up on pharmacy shelves as one of those common supplements. But when people spot a label with a name that long, questions start stacking up. So, what is ferric ammonium citrate, and is it truly safe for children?
Understanding What Ferric Ammonium Citrate Does
This compound acts as an iron supplement. Your body uses iron to make hemoglobin, the protein in red blood cells that helps move oxygen around. If a child doesn’t get enough iron in their diet, tiredness, weakness, and problems focusing follow close behind. Pediatricians often test for anemia by looking at a child’s hemoglobin levels. If they’re low, a supplement seems like the next logical step.
Paying Attention to Dosage and Side Effects
Doctors and pharmacists have seen ferric ammonium citrate help plenty of children with iron deficiency, but only at the right dose. Giving a child too much iron can cause stomach pain, vomiting, and sometimes more serious health risks. I remember the worry I felt once when my niece got into her mom’s iron pills. It sent her to the emergency room, but quick action prevented long-term problems. The lesson? Stick with the doctor’s dosing instructions, and always keep supplements up and away from little hands.
The most common side effects of iron supplements include black stools and stomach upset. These usually show up with pretty much any iron pill. The bigger issues come from taking more than directed over weeks or months, which stresses the liver and other organs. Parents should watch for symptoms such as severe abdominal pain, vomiting blood, or persistent constipation. These call for a trip to the doctor, no hesitation.
Checking the Evidence and Advice
Research studies generally support the safety of ferric ammonium citrate in kids—provided it’s given in the amounts recommended by the pediatrician. Health authorities like the US Food and Drug Administration, the NHS, and the World Health Organization consider this supplement effective for treating iron deficiency anemia. At the same time, professional organizations have made it clear that all iron supplements need careful monitoring.
Over the years, some families have raised concerns about additives and extra ingredients in children’s supplements. Looking at the ingredient list or asking the pharmacist helps with peace of mind. For households with kids who have history of allergies or special medical conditions, it helps to double-check with a specialist before starting any supplement.
Supporting Children’s Iron Health Safely
Balanced eating—lean meats, beans, leafy greens—still plays the biggest role in preventing iron deficiency. Some families deal with picky eaters or tight budgets, so supplements become part of the plan. Supervision, medical advice, and proper storage make the biggest difference in keeping kids safe.
Parents want to do right by their children. Ferric ammonium citrate can be a helpful solution for iron deficiency when used thoughtfully. Before starting or continuing any iron supplement, talking with your child’s doctor ensures safety and answers to any concerns.
How should Ferric Ammonium Citrate be stored?
Storing Chemicals Going Beyond the Basics
Working in labs has shown me that a small change in how a chemical is stored can turn a safe workspace into a risky one. Ferric ammonium citrate stands as a perfect example. Left exposed, this compound can degrade or even cause trouble. Simple steps can make all the difference in keeping both people and the material safe.
Why Precise Storage Makes a Difference
Ferric ammonium citrate breaks down easily with moisture and light. If humidity creeps into the container, it can start clumping or turning brown. Once that happens, assays for iron content produce odd results, and anyone relying on its properties in food or medicine runs into issues. Poor storage slowly erases trust in the material, along with the science built on top of it.
Looking back at my time as a lab technician, I remember two groups using ferric ammonium citrate for microbiology plates. One kept the jars tightly sealed, tucked away from light in a dry cabinet. Their results came back clean every run. The other group left lids loose, let light seep in, and kept supplies near the sink. Their samples spoiled within weeks. These subtle habits, invisible in an inventory log, decide whether a project succeeds or fails.
What Proper Storage Looks Like
Dryness ranks at the top of the list. Moisture in the air reacts with ferric ammonium citrate, cutting its shelf life short. Stored in airtight, moisture-proof containers, the product holds up much longer. Glass works better than plastic if the lid seals well and resists wear. Desiccants—those little silica gel packs many people throw out—can help soak up any stray humidity.
Direct sunlight or even bright indoor lighting can trigger changes, so cabinets that block light keep the compound stable. Painted steel cabinets or thick wooden cupboards do the trick, provided bottles go back on the shelf right after use.
Temperature swings also matter. Too much warmth pushes along chemical changes, but so does freezing, which can cause condensation once things warm back up. Most labs and storerooms set aside a specific area away from radiators and out of the way of airflow from air conditioners. Conditions that hover near room temperature, steady, without sharp jumps, prevent a lot of spoilage.
Clearly labeling containers with open dates, shelf life, and hazard warnings pays dividends down the line. Ferric ammonium citrate is safe to handle in small quantities, but skin contact and inhaling dust should always be avoided. Gloves, goggles, and dust masks help, especially during transfers between storage and workspace. Disposal instructions also belong nearby, since tossing chemicals in the trash or pouring them down the sink can cause environmental trouble.
Tweaking Habits for Better Results
It’s tempting to cut corners in small, routine actions—pushing the bottle back on the shelf unsealed or ignoring the spill in a rush. Every time one of those shortcuts is taken, risk creeps in. Training, regular reminders, and visible storage guidelines steer teams toward good habits. In workplaces where accidents have happened, new standards usually land fast. I’ve seen more than one lab switch to locking cabinets and labeled logs after a single scare.
Handling ferric ammonium citrate with a bit of respect protects not just the science but the entire team. Simple safeguards, taken seriously, protect budgets, results, and people. Every small step given to storage echoes through results and safety. Attention in storage, as any seasoned chemist will say, pays off far beyond the few seconds it takes to do things right.
Can Ferric Ammonium Citrate interact with other medications?
Why Ferric Ammonium Citrate Stands Out
Ferric ammonium citrate pops up in the lives of folks managing iron deficiency or certain medical tests. Many recognize it as a supplement, but its basic job, delivering iron, means it doesn’t play well with every medicine. Navigating its place among other daily pills and treatments can take some attention.
Mixing Iron with Other Drugs: Real-World Considerations
Taking ferric ammonium citrate with certain medications easily changes how well those drugs work. Calcium, magnesium, and antacids, for example, crowd out iron in the stomach, making it tougher for the body to grab onto ferric ammonium citrate. Combining iron supplements with antibiotics like tetracyclines or fluoroquinolones leaves both drugs struggling—iron can lower the strength of the antibiotic, and the antibiotic can stop the body from absorbing iron properly.
Not only antacids and antibiotics pose a risk. Medications for thyroid conditions—including levothyroxine—sometimes react to iron, messing with hormone levels. I’ve seen plenty of people surprised by low thyroid hormone results, only to track the problem back to an innocent-looking iron pill. As a person who has helped parents and older adults manage multiple prescriptions, keeping an eye on such combinations proves vital if you care about getting the most out of your treatments.
Food, Drink, and Supplements—Where Interactions Slip In
Even without other drugs on board, ferric ammonium citrate’s performance can drop with the wrong mix of foods. Coffee, tea, many dairy products, and high-fiber cereals usually get in the way of iron’s ride into the bloodstream. Timing meals, snacks, and daily medications makes a difference for people trying to raise or steady their iron stores.
Vitamin C helps iron get absorbed, so squeezing in a glass of orange juice alongside ferric ammonium citrate often lifts results. But timing matters more than most people think. Spacing out doses of iron and other tricky meds by about two hours usually steers clear of the worst clashes.
Stories from the Clinic: The Value of Practical Advice
In my time working with older folks juggling multiple prescriptions, I’ve watched people struggle with fatigue or stomach issues, only to discover an iron supplement was awkwardly scheduled with other pills. For many, swapping the order of breakfast, coffee, and medicines gave them more energy and improved their blood counts. Others saw stubborn infections clear up after adjusting when they took their antibiotics and iron. These small changes, suggested by pharmacists or attentive doctors who spot patterns in lab results, stick out in my memory because they brought real, positive changes in people’s daily lives.
Smart Solutions and Teamwork
Managing these interactions takes teamwork among patients, doctors, and pharmacists. Doctors want to avoid clashes, and pharmacists catch errors, but patients hold the power to share what they actually take and when. Writing down every medicine, supplement, and vitamin you take helps everyone. It also pays to ask direct questions about timing and drug combinations—no one expects patients to memorize all possible interactions.
Technology helps too. Pill reminder apps and digital health records make it easier to keep up with the schedule. Pharmacists with up-to-date interaction checkers on their computers have flagged many risky combinations before trouble starts. Sharing updates from every new prescription means fewer side effects, better health, and fewer wasted days.
Staying Informed—Why It Matters
Anyone taking ferric ammonium citrate with other meds stands a better chance of getting the benefits and avoiding trouble by knowing how these pills interact. Health sites run by major hospitals, advice from pharmacists, and detailed medication guides keep the facts close at hand. The more you ask, read, and track, the easier it gets to dodge the setbacks and feel better while taking care of iron needs.
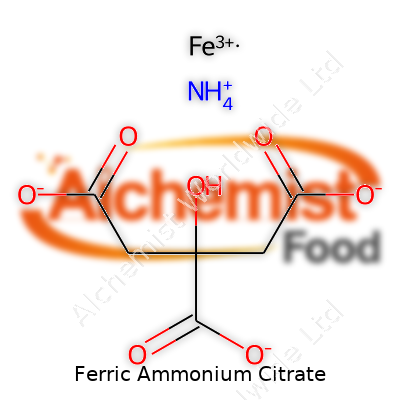
| Names | |
| Preferred IUPAC name | Iron(3+) ammonium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Ammonium iron(III) citrate Ammonium ferric citrate Ferric ammonium citrate brown Ferric ammonium citrate green |
| Pronunciation | /ˈfɛr.ɪk əˈmoʊ.ni.əm ˈsɪt.reɪt/ |
| Preferred IUPAC name | Ammonium iron(3+) 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Ammonium ferric citrate Ferric ammonium citrate green Iron ammonium citrate |
| Pronunciation | /ˈfɛrɪk əˈmɒniəm ˈsɪtrət/ |
| Identifiers | |
| CAS Number | 1185-57-5 |
| Beilstein Reference | 1722340 |
| ChEBI | CHEBI:31624 |
| ChEMBL | CHEMBL1201772 |
| ChemSpider | 23722 |
| DrugBank | DB09146 |
| ECHA InfoCard | 03b4e382-fd7b-4246-8000-5795311cc6e7 |
| EC Number | 231-753-5 |
| Gmelin Reference | 77818 |
| KEGG | C02345 |
| MeSH | D005247 |
| PubChem CID | 159729 |
| RTECS number | JO6300000 |
| UNII | T0Y3286KZN |
| UN number | UN3077 |
| CAS Number | 1185-57-5 |
| Beilstein Reference | 4029207 |
| ChEBI | CHEBI:31637 |
| ChEMBL | CHEMBL1201537 |
| ChemSpider | 5013547 |
| DrugBank | DB14472 |
| ECHA InfoCard | 100.028.282 |
| EC Number | 231-753-5 |
| Gmelin Reference | 13768 |
| KEGG | C01438 |
| MeSH | D005247 |
| PubChem CID | 159729 |
| RTECS number | QU8750000 |
| UNII | F93TZV843E |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | urn:epa.comptox.dashboard:DTXSID2020905 |
| Properties | |
| Chemical formula | C6H8FeNO7 |
| Molar mass | 449.14 g/mol |
| Appearance | Dark red brown crystals or granules |
| Odor | Odorless |
| Density | 1.684 g/cm³ |
| Solubility in water | Very soluble in water |
| log P | -4.0 |
| Acidity (pKa) | 3.1 |
| Basicity (pKb) | 8.7 |
| Magnetic susceptibility (χ) | +3500e-6 cm³/mol |
| Viscosity | Viscous liquid |
| Dipole moment | 0 D |
| Chemical formula | C6H8FeNNaO7 |
| Molar mass | 449.14 g/mol |
| Appearance | Dark reddish brown to yellowish brown, granular powder or crystals |
| Odor | Odorless |
| Density | 1.684 g/cm³ |
| Solubility in water | Very soluble in water |
| log P | -4.0 |
| Vapor pressure | <1 mm Hg (25 °C) |
| Acidity (pKa) | 3.1 |
| Basicity (pKb) | 8.7 |
| Magnetic susceptibility (χ) | 'Antiferromagnetic' |
| Dipole moment | 1.01 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 259 J·mol⁻¹·K⁻¹ |
| Std molar entropy (S⦵298) | 192 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | B03AA02 |
| ATC code | B03AB04 |
| Hazards | |
| Main hazards | May cause respiratory irritation, eye irritation, and skin irritation |
| GHS labelling | GHS07, GHS09, Warning, H315, H319, H335, H410 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "Wash skin thoroughly after handling. Do not eat, drink or smoke when using this product. IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth. |
| Lethal dose or concentration | LD50 oral rat 1,570 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 1,730 mg/kg |
| NIOSH | SN1575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Ferric Ammonium Citrate: Not established |
| REL (Recommended) | 20 mg/kg bw |
| IDLH (Immediate danger) | Not listed |
| Main hazards | Harmful if swallowed, causes skin and eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Wash thoroughly after handling. Do not eat, drink or smoke when using this product. |
| NFPA 704 (fire diamond) | 1-0-1 |
| Lethal dose or concentration | LD50 oral rat 3,300 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 3250 mg/kg |
| NIOSH | WH7300000 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 5 mg |
| Related compounds | |
| Related compounds |
Ferric citrate Ammonium citrate Iron(III) salts Citric acid |
| Related compounds |
Ferric citrate Ammonium citrate Iron(III) salts Ammonium iron(III) sulfate Ferrous ammonium sulfate |

