Ethyl Arachidonate: A Close Look at a Unique Lipid Compound
Historical Development
Ethyl arachidonate didn’t always get center stage in research labs. Interest picked up after scientists started diving into the role of fatty acids in our bodies. Around the late 20th century, more attention went toward molecules like arachidonic acid and its derivatives due to their links to inflammation and cell signaling. As medical research advanced, folks realized these compounds played real parts in biological systems. Ethyl arachidonate, a simple ethyl ester derivative, saw rising demand once researchers saw opportunities for modifying natural fats to study both their biological roles and their potential in industrial scenarios. Its development mirrored the shift from basic chemistry toward tasks like drug delivery and studying disease markers, highlighting a trend where chemical derivatives often open doors to deeper understanding and practical solutions.
Product Overview
Ethyl arachidonate stands out as an ester formed by combining ethanol and arachidonic acid. You find this molecule mostly in scientific circles, where chemists and biologists use it to explore metabolic pathways and drug metabolism. In practical terms, it acts as a model compound, a building block, and, at times, a surrogate for studying how the body handles certain lipids. Commercial interest isn’t as broad as for other esters. Most demand comes from research, analytical method development, and, occasionally, specialty nutrition and cosmetic fields. As a research-grade product, it arrives with strict purity standards, since even small impurities can throw off experimental results.
Physical & Chemical Properties
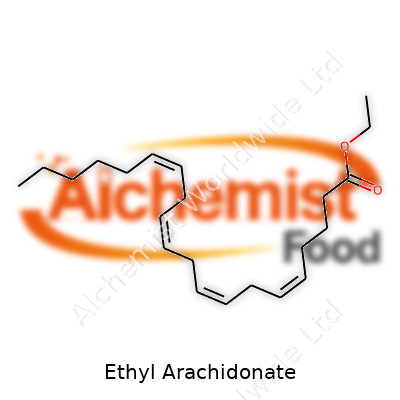
This compound comes as a colorless to pale yellow, oily liquid. Its molecular formula, C22H36O2, leads to a molecular weight of around 332.5 g/mol. Its structure features a twenty-carbon chain with four cis double bonds—typical of arachidonic acid— finished off as an ethyl ester instead of a carboxylic acid. It resists mixing with water, but dissolves well in organic solvents like ethanol, chloroform, or hexane. Melting points sit below room temperature, and the boiling point lies considerably higher. Since it’s a polyunsaturated fat, exposure to air and light sets up the scene for oxidation, which means proper storage in amber bottles and refrigeration makes a big difference. Unlike many fats and oils, the esters form less stable emulsions and react differently during processing or formulation.
Technical Specifications & Labeling
Quality comes down to purity—above 98% in most labs—verified with gas chromatography or high-performance liquid chromatography. Labels highlight the batch number, storage conditions (usually stating below 0°C and away from light), hazard codes, and the usual reminders found with chemicals of this type. The product’s traceability links right back to original production lots, which helps track any inconsistencies that might surface in downstream research. Any vendor shipping this to a laboratory needs to provide a certificate of analysis with real data, making it easier for scientists to compare expected outcomes with actual results. Some labels go further, mentioning major impurities like free arachidonic acid or residual solvents.
Preparation Method
Producing ethyl arachidonate starts with the esterification of arachidonic acid using ethanol, usually in the presence of an acid catalyst. Classic Fischer esterification gets the job done in most lab setups, though scaling up for larger batches might push toward more efficient, less water-sensitive methods. After reacting, chemists typically rely on liquid-liquid extraction to pull out excess ethanol and unreacted acid, followed by purification with distillation or column chromatography. Careful handling of reagents matters since the many double bonds in the chain make the molecule prone to side reactions, including oxidation and isomerization. Choosing antioxidants and controlling oxygen exposure during production can preserve product integrity.
Chemical Reactions & Modifications
Once in hand, ethyl arachidonate acts as a launchpad for a range of chemical tweaks. Hydrogenation straightens out its double bonds, sometimes used to experiment with fully saturated analogs. Oxidation produces epoxides or hydroxyl groups, mimicking metabolites formed in living systems. Base-catalyzed hydrolysis flips the compound back to arachidonic acid, a reaction used by biochemists investigating enzyme activities or metabolic pathways. Halogenation, chain shortening, or even tagging with stable isotopes allows for tracking these molecules during metabolic studies. These reactions not only help synthesize new materials but also unpack mysteries of cell membranes and signaling compounds in actual human tissues.
Synonyms & Product Names
You’ll spot this compound as arachidonic acid ethyl ester or just ethyl 5,8,11,14-eicosatetraenoate in chemical catalogs. In specialty databases, it might appear as ethyl (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoate. These synonyms make tracking information across different regions and industries less of a guessing game, especially since country-specific regulations use different naming schemes. For commercial invoices, shorter labels—like EAEA or simply ethyl arachidonate—tend to show up, sometimes with the corresponding CAS number to clear up any confusion.
Safety & Operational Standards
Handling ethyl arachidonate takes a basic knowledge of lab safety. As an ester with multiple double bonds, it doesn’t present major acute toxicity concerns, but you still want to avoid getting it on your skin or eyes. Gloves, goggles, lab coats, and good ventilation all matter more than most folks expect for ‘simple’ organic liquids. The big concern comes from oxidative products that can form dioxins or other hazardous byproducts if the substance gets overheated or degraded. Waste handling falls under standard organic disposal rules, but chemical hygiene plans should recognize the possibility of peroxide formation. Any workplace handling this material ought to keep spill kits, fire extinguishers, and appropriate ventilation at hand.
Application Area
Research teams rely heavily on ethyl arachidonate to model metabolic processes, especially for enzymes that process polyunsaturated fatty acids. Pharmacologists put it to work designing studies on lipid digestion, bioavailability of supplements, and drug metabolism. Analytical chemists use it as a standard for calibrating instruments that measure fatty acids in tissues or foodstuffs. Some secondary uses show up in nutritional science, where modified esters provide controlled-release forms of fatty acids for dietary studies. Cosmetic research sometimes explores its effects on skin barrier properties or absorption. While you won’t find it on supermarket shelves or in mass-market skin creams, its reach stretches across several disciplines eager to unravel the complicated world of fatty acid biochemistry.
Research & Development
Most new developments involving ethyl arachidonate tie back to life sciences and analytical method platforms. Teams in medical research keep looking for clearer markers of health states linked to fatty acid pathways, using labeled esters to trace how molecules move and break down in the body. Drug developers push for better delivery vehicles that mimic endogenous lipids, and ethyl arachidonate offers a step up from relying only on glycerides. Advances in lipidomics—where researchers map out hundreds of fatty acid derivatives—come to life partly because standard compounds like this one let different teams calibrate and compare results. Academic and corporate labs keep refining both the ways they make the compound and the ways they harness it, from large-scale synthesis up through minute-by-minute tracking inside living cells.
Toxicity Research
Toxicologists don’t sound much alarm about ethyl arachidonate under controlled conditions. Oral ingestion or skin contact in small, lab-scale amounts has not triggered major adverse effects, though large doses used to study acute or chronic toxicity can reveal subtle issues. Breakdown products—especially after exposure to high heat or oxidative conditions—raise more concern, with some studies highlighting oxidative stress and inflammation as possible outcomes in animal models. Standard safety reviews in the literature report low risks under traditional handling, but experts point out the growing gap between what happens in a test tube and real-world exposure scenarios, especially considering nanoparticles, emulsions, or new delivery systems. Long-term research keeps an eye on environmental persistence and metabolism, since fatty acid derivatives like this can move through aquatic and soil systems in ways that outpace earlier chemical assumptions.
Future Prospects
Ethyl arachidonate looks set to play a growing part in the fields of lipidomics, pharmacology, and specialty nutrition. As more health studies uncover the interconnected impacts of dietary fats, researchers push for deeper understanding of metabolic pathways—an area where clean, stable, well-characterized standards shine brightest. Rapid advances in instrument technology, including mass spectrometry and advanced chromatography, drive up demand for reliable reference compounds. Meanwhile, as synthetic biology pushes the boundaries of custom fats and oils for both food and drug uses, companies experimenting with modified lipids return over and over to simple, scalable building blocks. Continued inquiry into inflammation, metabolic disease, and precision nutrition keeps ethyl arachidonate near the top of the list for new projects and technology platforms. Real progress comes not from a single molecule, but from the collective ability to track, model, and harness it in systems that improve both our understanding and quality of life.
What is Ethyl Arachidonate used for?
Understanding Ethyl Arachidonate
Ethyl arachidonate comes from arachidonic acid, which shows up in the body as an omega-6 fatty acid. It’s the sort of name you’d expect to find in a specialist’s handbook, but plenty of attention in recent years has fallen on this compound. You’ll see ethyl arachidonate used mostly as a chemical building block in both the pharmaceutical and research fields. The world of fatty acids plays a crucial role in health, which explains why researchers keep such a close eye on ethyl arachidonate’s effects and possible applications.
Medical and Research Applications
Scientists rely on ethyl arachidonate for laboratory experiments related to inflammation, cell growth, and nerve health. Researchers look into how the body processes fatty acids by using ethyl arachidonate as a model substance. This compound gives them a controlled way to study metabolic reactions and cell signaling pathways—key for understanding conditions like Alzheimer’s disease or chronic inflammation.
Drug developers have explored ethyl arachidonate’s potential for new medications designed to target inflammation or help with neurological issues. A researcher friend of mine once spent months testing how different esters of fatty acids, including ethyl arachidonate, influenced the healing processes in animal tissue samples. His work pointed to modest effects on inflammation, a crucial factor in everything from joint pain to heart disease.
Possible Side Effects and Safety
Ethyl arachidonate draws attention because the human body responds strongly to changes in fatty acid intake. Too much of certain fatty acids can tip the scales and worsen inflammation instead of helping it. People with backgrounds in clinical nutrition watch how these compounds interact since the wrong balance can cause more harm than good. Authorities such as the FDA have not approved its use for regular supplementation or medication, meaning any handling needs care and a strong understanding of risk. The value sits mainly in laboratory research and not in over-the-counter products.
Challenges and Responsible Research
Many critics argue that the rush to test new fatty acid derivatives often overlooks long-term safety data. This creates a gap where possible benefits never fully reach the public, or—on the flip side—side effects catch researchers off guard. I’ve seen more than one study forced to pull back because the compound, when moved from test tubes to living systems, brought unexpected results.
Moving Forward: Possible Solutions
Ethyl arachidonate shows promise, but safe and informed use stands as the top priority. Improved transparency in laboratory testing would help the wider scientific community share both mistakes and breakthroughs. Encouraging more open access to research data addresses some of the trust issues around new chemical applications. Regulatory bodies should keep asking tough questions about purity, dosage, and long-term exposure. Universities and research labs gain more from collaboration than from siloed investigation. With better communication between clinicians, chemists, and regulators, both safety and discovery move ahead faster. As scientists uncover more about how fatty acids work, careful oversight and collaboration will make sure that these lessons actually help people—not just fill up more pages in academic journals.
Is Ethyl Arachidonate safe for human consumption?
What You Should Know About Ethyl Arachidonate
Food and supplement companies keep reaching for new ingredients hoping to boost health claims, protein content, or market buzz. Ethyl arachidonate has turned up in discussion, mainly because it’s related to arachidonic acid, a fatty acid already present in some foods and the human body. The question is simple: is it safe?
Understanding Ethyl Arachidonate
Chemically, ethyl arachidonate is an ester made by combining arachidonic acid and ethanol. In the body, you find arachidonic acid in cell membranes—especially in the brain, muscles, and liver. You get some from eating meat, eggs, or certain fish. Some athletes and supplement brands highlight the supposed “muscle-building” effects of arachidonic acid, thinking its involvement in inflammation could help muscle growth. Manufacturers sometimes turn acid versions into esters to increase stability or absorption. That’s where ethyl arachidonate comes in.
What Research Tells Us
No credible long-term human trials stand out for ethyl arachidonate. Most research focuses on arachidonic acid itself, looking at safety and function in humans. In a handful of studies, doses of arachidonic acid around 1-1.5 grams per day didn’t show serious adverse effects. Still, regulators and scientific panels—including the European Food Safety Authority (EFSA)—limit these findings to the acid, not its ethyl ester.
Esters sometimes break down in the digestive tract, but not always at predictable rates. Your body may absorb an ethyl ester differently than it does the acid. For example, fish oil supplements that contain ethyl esters get absorbed less efficiently than the natural triglyceride forms. If you want proof on safety, you need good studies specifically about ethyl arachidonate, not just guesses from related compounds.
Unknowns Bring Real Risks
Any new food additive or supplement ingredient deserves real scrutiny before hitting the shelves. No government food agency in the United States, Europe, or Japan has approved ethyl arachidonate as a food additive or supplement. If it shows up in a product, it’s likely being marketed in a legal gray area. Without approval, you don’t have standards for purity, strength, or safe dosage.
From a practical perspective, adding new esters of fatty acids carries unpredictable effects on health. Arachidonic acid itself can fuel inflammation; in healthy bodies, that’s essential for fighting infection or healing injuries. Go too far, and chronic inflammation ties to problems like heart disease, arthritis, and mood disorders. People with asthma, autoimmune illnesses, or high cardiovascular risk may face heightened trouble if the body pulls in extra arachidonic acid from supplements or new additives.
What Informed Consumers Can Do
Knowledge can save you time, money, and headaches. Check ingredient lists, and ask companies for documentation if they are using unfamiliar compounds like ethyl arachidonate. Companies that care about safety prove it with data. You deserve to know where an ingredient comes from and what it might do in the body.
Research before trying a supplement, and ask your healthcare provider—especially if you’re at risk of heart issues, allergies, or inflammatory conditions. If in doubt, leave it out. Nutrition comes from a variety of whole foods, not shortcuts built on synthetic additives waiting for research to catch up.
What are the potential side effects of Ethyl Arachidonate?
Understanding Ethyl Arachidonate
Ethyl arachidonate sounds like just another complicated chemical, but at the bottom of it, we’re talking about a derivative of arachidonic acid—a fatty acid people usually get from meat, eggs, and some fish. Its close ties to the omega-6 family may make it attractive in research circles, but adding it to diets or supplements isn’t something anyone should do without giving potential drawbacks a real look.
What Can Go Wrong with Too Much Omega-6?
Your body needs some omega-6, including arachidonic acid, for normal brain function and cell growth. The trouble starts when the ratio of omega-6 to omega-3 gets out of whack. Loading up on products containing ethyl arachidonate raises that risk. Studies link high levels of omega-6 to more inflammation in your body. Inflammation isn’t just about the occasional stiff joint—it can push up chances of heart disease, certain cancers, and autoimmune flare-ups. So chasing health by tossing new fatty acids into the mix sometimes backfires.
Stomach Upset and Digestion
Whenever labs tinker with fatty acids, upset stomach follows close behind. Nausea, diarrhea, and indigestion pop up in people who take high doses of any fatty acid ester, including ethyl arachidonate. These reactions aren’t just inconvenient—they cut into quality of life and make it harder to stick with any wellness routine. Some folks even report abdominal pain and cramping if their systems don’t agree with the chemical changes.
Hormone and Immune System Concerns
Ethyl arachidonate feeds directly into the production of prostaglandins. In plain terms, these messengers control processes like swelling, pain, and even smooth muscle contraction. If levels swing too high, women may see changes in menstrual cycles or experience worse cramps due to more prostaglandins. Overstimulation of the immune system also leaves people more sensitive to allergies or skin problems like eczema. These aren’t fringe concerns—doctors track prostaglandin impact every day in patients with chronic medical issues.
Other Side Effects Under Study
Animal trials and smaller human studies raise even more flags. Elevated liver enzymes have shown up in some participants, hinting at potential liver stress, especially in those with underlying liver concerns. Lab data occasionally finds changes in kidney function and blood pressure, likely tied to shifts in fluid regulation. In the hands of healthy adults, these side effects might not jump off the page. That picture changes for children, pregnant people, and anyone managing kidney or liver conditions.
What Can People Do About These Risks?
Before starting supplements with ethyl arachidonate or any omega-6 variant, a conversation with a healthcare professional is crucial. There’s still not enough long-term safety data. Standard advice—balance omega-3 and omega-6 intake, aim for whole foods, and avoid sudden dietary shifts on your own—applies more than ever. If symptoms like tummy pain, fatigue, or skin changes show up, dialing back intake and getting tested for liver enzyme levels doesn’t hurt.
The Research Continues
Researchers continue studying both the good and bad of fatty acid esters. What we know so far: high-dose or long-term use of ethyl arachidonate triples the need for careful oversight. With more clinical trials, the medical field may someday pinpoint the safest ways to use these compounds, but the public shouldn’t get ahead of the science.
Sources Matter
Trustworthy supplements display clear sourcing and dosing information. Products with pharmaceutical-grade raw materials, transparent manufacturing practices, and batch tests for purity guard against contamination or unexpectedly high concentrations.
How should Ethyl Arachidonate be stored?
Keeping Quality in Mind
Ethyl arachidonate doesn’t get much attention unless someone deals with specialty chemicals or advanced labs. Yet, this compound isn’t just another bottle on a shelf. Lab-grade ingredients can break down or turn useless without proper handling. A few years ago, I left an old bottle of ethyl arachidonate exposed on a cluttered workbench. After a month, that sample turned yellow, smelled a bit funny, and had lost the consistency we needed for our next batch. That’s the kind of mistake that eats up budget and time.
Oils and esters with unsaturated bonds, like ethyl arachidonate, react with oxygen, light, and heat. Even small leaks in caps or careless transfers can introduce contaminants. Storing it wrong pushes it to oxidize, degrade, or create byproducts, wrecking its performance, whether used in research or as an ingredient.
What Science Says About Storage
Best practice for this compound: keep it tightly sealed in an amber glass bottle. Sunlight has enough energy to kick off unwanted chemical reactions, especially for oils with polyunsaturated chains. Research from chemical safety guides published by Sigma-Aldrich and TCI show that room temperature storage, out of direct sunlight, helps keep things stable. Keeping it at 2-8°C in a standard refrigerator can slow down any reactions even more. Most labs stash sensitive oils this way, and from personal experience, nothing beats consistency for quality.
Moisture also matters. Even small amounts of water creep inside a loosely closed bottle, spurring hydrolysis. Over time, this means you get free fatty acids and alcohol, which undermines projects that demand purity—whether pharmaceutical or analytical. To cut this risk, storing ethyl arachidonate in a desiccator scores better protection, especially if you run a humid climate or your fridge collects condensation.
Practical Moves to Avoid Spoilage
Support staff and researchers sometimes rely too much on labeling and forget about the nitty-gritty. Here’s what keeps ethyl arachidonate in good shape:
- Always keep the bottle capped snug after every use.
- Label each bottle with the date it was opened, not just the receipt date. Chemicals age whether you use them or not.
- If breaking up into smaller portions, use clean, dry amber vials to avoid introducing extra air or moisture.
- Don’t let this compound sit close to heating vents or power supplies—they crank the temperature up enough to cause slow degradation over weeks or months.
- If you’re in a big facility, place a clear sign or info card about sunlight and temperature near the refrigerated section holding oils and esters.
Manufacturers and suppliers often stamp lots with expiration dates, but in real-world labs, actual quality boils down to careful storage and common sense. Once you spot color changes, off odors, or cloudiness, toss the sample instead of risking bad results.
Smart Solutions for the Long Haul
Invest in simple chemical-resistant storage cabinets away from direct sun if you don’t have a designated refrigerator. Staff turnover can cause careless mistakes, so routine checks make a difference—train every new lab member on the storage policy. Buying only what you’ll use within a few months cuts down on old stock lingering around and going bad. Facilities with high humidity might look to low-cost silica gel packs in storage cabinets for an extra layer of defense. These everyday steps protect budgets and reputations, ensuring each shipment of ethyl arachidonate delivers what buyers expect.
What is the recommended dosage for Ethyl Arachidonate?
Understanding Ethyl Arachidonate
Most people run into ethyl arachidonate through medical or research circles, usually in conversation about fatty acids, inflammation, or nutritional supplements. Scientists isolate this compound from arachidonic acid, an omega-6 polyunsaturated fatty acid found in cell membranes and some foods. It shows up in experimental protocols and has caught the attention of supplement companies chasing the next big thing.
Digging Into Recommended Dosage
Right off the bat, it's important to point out that doctors and standard-setting organizations have not established any recommended dosage for ethyl arachidonate for the general population. No recognized pharmacopoeia or regulatory body, like the FDA or European Medicines Agency, provides daily limits, therapeutic targets, or upper safety thresholds for this substance. That leaves anyone curious about trying it without a clear roadmap from trusted authorities.
In the lab, researchers sometimes use doses that range from micrograms per kilogram to a few milligrams per kilogram, depending on the animal or cell line and the purpose of the test. Human studies are rare. Most data come from models that do not always translate cleanly to people. Sometimes a published paper might mention results after feeding test animals a preparation containing ethyl arachidonate, but scaling that for use in humans is risky business. Factors like absorption, metabolism, and interaction with medications or disease conditions make all the difference.
Safety, Side Effects, and Oversight
No robust record of controlled clinical trials exists for ethyl arachidonate. No one has collected enough evidence to claim that taking any dose is safe over a sustained period. Some people look at fatty acid research in general and assume benefits or safety, but that can be misleading. Not all fatty acids act the same, and the body sometimes processes an ethyl ester differently from the acid itself. For example, some esters might stay in your blood longer, leading to effects no one expects or wants.
Self-experimentation always carries risks. Unpredictable reactions, allergic responses, and changes in enzyme activity can catch people off guard. No supplement, no matter how closely related to a well-known nutrient, can guarantee the same results or safety profile. This is especially true for niche or research-grade chemicals like ethyl arachidonate, which may contain impurities or be formulated for purposes far removed from human consumption.
Pushing for Evidence, Not Anecdote
It's tempting to look for shortcuts to health using novel supplements, especially those linked to hot areas like inflammation reduction or cognitive enhancement. But personal experience and anecdotes give a distorted picture, especially without large-scale studies to confirm benefits or risks. This is where sticking to evidence-based guidelines matters.
Anyone tempted to try ethyl arachidonate should check in with a healthcare professional who knows their medical history. Sometimes, the promise of a compound in a research setting does not translate into real-world benefit. Clear communication and skepticism can save a lot of trouble down the road.
Potential Solutions and Responsible Approaches
Better oversight and research will help answer questions about ethyl arachidonate. Pushing for well-controlled, peer-reviewed studies could clear up the safety profile and maybe lead to real recommendations. Until then, supplement seekers should resist experimenting solo and rely more on tried-and-true approaches for whatever they're hoping to improve, whether it's joint health, heart function, or brain performance. Experience suggests that patience, curiosity, and a solid foundation of evidence always serve better than hope and hype.
| Names | |
| Preferred IUPAC name | Ethyl (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoate |
| Other names |
ETHYL 5,8,11,14-EICOSATETRAENOATE ETHYL ARACHIDONATE ARACHIDONIC ACID ETHYL ESTER ETHYL icosa-5,8,11,14-tetraenoate |
| Pronunciation | /ˌɛθɪl əˌrækɪˈdəʊneɪt/ |
| Preferred IUPAC name | ethyl (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoate |
| Other names |
Arachidonic acid ethyl ester Ethyl eicosatetraenoate Ethyl 5,8,11,14-eicosatetraenoate |
| Pronunciation | /ˌiːθɪl əˌrækɪˈdoʊneɪt/ |
| Identifiers | |
| CAS Number | 3032-55-1 |
| Beilstein Reference | 1843184 |
| ChEBI | CHEBI:53299 |
| ChEMBL | CHEMBL504203 |
| ChemSpider | 83557 |
| DrugBank | DB14098 |
| ECHA InfoCard | 100.192.302 |
| EC Number | 206-962-3 |
| Gmelin Reference | Gmelin Reference: **8323** |
| KEGG | C16147 |
| MeSH | D020928 |
| PubChem CID | 5282760 |
| RTECS number | RN2104K040 |
| UNII | 1U8QVS994M |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | `DTXSID0026016` |
| CAS Number | 63831-20-7 |
| 3D model (JSmol) | `/showmol.php?id=C22H36O2&model=ball&format=jsmol` |
| Beilstein Reference | 1462460 |
| ChEBI | CHEBI:52757 |
| ChEMBL | CHEMBL473425 |
| ChemSpider | 140678 |
| DrugBank | DB14057 |
| ECHA InfoCard | 100.165.304 |
| EC Number | 204-821-1 |
| Gmelin Reference | Gmelin Reference: **81734** |
| KEGG | C08544 |
| MeSH | D000073566 |
| PubChem CID | 5282761 |
| RTECS number | AT2625000 |
| UNII | B7KQ96I45Q |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | Q27125394 |
| Properties | |
| Chemical formula | C22H36O2 |
| Molar mass | 318.51 g/mol |
| Appearance | Colorless to light yellow liquid |
| Density | 0.922 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 4.82 |
| Vapor pressure | <0.0001 mmHg (20°C) |
| Acidity (pKa) | 4.75 |
| Basicity (pKb) | 13.96 |
| Refractive index (nD) | 1.475 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.0478 D |
| Chemical formula | C22H36O2 |
| Molar mass | 346.57 g/mol |
| Appearance | Colorless to light yellow oily liquid |
| Odor | Odorless |
| Density | 0.95 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 4.6 |
| Vapor pressure | 0.0000116 mmHg at 25°C |
| Acidity (pKa) | 4.75 |
| Refractive index (nD) | 1.470 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.55 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.1 J·mol⁻¹·K⁻¹ |
| Std molar entropy (S⦵298) | 589.9 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | A05AD03 |
| ATC code | A05AD02 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P501 |
| Flash point | 193°C |
| Autoignition temperature | 400°C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 25 mg |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P272, P273, P280, P302+P352, P305+P351+P338, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 161 °C |
| Lethal dose or concentration | LD50 oral rat 28200 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | General laboratory chemicals |
| Related compounds | |
| Related compounds |
Arachidonic acid Methyl Arachidonate Arachidonoyl chloride Arachidonoylethanolamide (AEA) Docosahexaenoic acid (DHA) |
| Related compounds |
Arachidonic acid Methyl arachidonate Arachidonoyl chloride Arachidonamide Arachidonoyl ethanolamide |

