Ethoxyquinoline: Perspectives from Bench to Benchmarks
Historical Development
Science leaves a detailed map when a compound first steps onto the scene. Ethoxyquinoline came about during a wave of heterocyclic chemistry research. In the twentieth century, chemists looked to quinoline rings for everything from dye production to anti-malarial medicine, and explored modifications for industrial benefits. Ethoxyquinoline entered the record at a point when research focused on chemically modifying aromatic heterocycles, driven by a need for more robust antioxidants and specialty intermediates. Early records from patent archives and journals speak to persistent interest in its substituent patterns, with chemists tracking how an ethoxy group might tweak the reactivity and biological profile of the quinoline scaffold. Developments branched between chemical synthesis advances and application studies, reflecting a larger trend of turning to specialized aromatic compounds for improved performance or safety in commercial products.
Product Overview
Ethoxyquinoline grabs attention in specialty chemicals catalogues and research publications for its versatility. Its presence spans several industries—chemistry labs, pharmaceutical intermediates, agrochemical development, and material science. Product forms include powder and crystalline solid, usually off-white to yellow, depending on purity and source. Laboratories favor it for multi-step syntheses and as a molecular scaffold. Suppliers list it with a range of purity specifications, signaling tailored production for either exploratory research or manufacturing pipelines. In daily use, it helps drive reactions, acts as a functional additive, or builds complexity in target molecules.
Physical & Chemical Properties
A molecule’s actual in-hand behavior directs its applications. Ethoxyquinoline boasts a stable aromatic core, with the ethoxy group altering both its solubility and electron distribution. General melting points range typically between 60-90°C, with a clear deviation if side groups shift or impurities creep in during synthesis. This compound dissolves readily in organic solvents—think ethanol, chloroform, ether—while showing poor solubility in water, which concentrates handling in organic media. Its moderate volatility and faint aromatic odor echo the experience with related quinoline derivatives. Chemically, the quinoline skeleton welcomes substitutions, yet the ethoxy function lends a tweak to basicity and electrophilic behavior, often considered in planning downstream reactions.
Technical Specifications & Labeling
Lab bottles bear detailed labels—CAS number, molecular formula, molecular weight, lot number, purity percentage, and recommended storage conditions. Reputable suppliers support claims with third-party analytical data: NMR spectra, HPLC purity reports, and moisture content certifications. Companies involved in scale-up pay attention to batch reproducibility, trace contamination, particle size distribution (if relevant), and shelf life. Packaging typically avoids light and moisture exposure, as both can threaten chemical integrity, especially for research-scale volumes. Larger-scale producers may include regional compliance marks, including REACH registration for Europe or TSCA reporting in the United States, guiding shipment and storage requirements.
Preparation Method
Most often, ethoxyquinoline reaches the lab bench through Friedländer or Skraup-type cyclizations, integrating an ethoxy group onto the established quinoline ring system via nucleophilic aromatic substitution, alkylation, or condensation strategies. Synthesis routes balance cost, yield, and safety, with chemists leveraging known quinoline intermediates and reagents like ethyl halides or alcohols under acid or base catalysis. Post-synthesis steps include careful purification—typically crystallization or column chromatography—and thorough drying. Scale-up for bulk supply involves process adjustments for cost, waste minimization, and manageable reaction times, reflecting decades of organic synthesis know-how and constant improvement.
Chemical Reactions & Modifications
A chemist sees ethoxyquinoline not just as a molecule, but as a launchpad. Its aromaticity and available positions support diverse reactions: electrophilic aromatic substitution (like nitration or sulfonation), metal-catalyzed cross-coupling (e.g., Suzuki or Buchwald-Hartwig aminations), or further alkylations and oxidations. Removing or altering the ethoxy group opens new branches for derivative development and structure-activity studies, especially in pharmaceutical research. Chemical handling requires knowledge of sensitivity—harsh acids or bases can sometimes cause unwanted decomposition or rearrangement. In hands-on experience, adapting reaction conditions proves crucial for high yields and clean transformations, as the ethoxy substituent can shuffle electronic properties enough to demand tailored protocols.
Synonyms & Product Names
Tracking a compound across industries can feel like chasing a chameleon. Ethoxyquinoline sometimes appears under alternative names, including 2-ethoxyquinoline or ethoxy-quinoline, pinpointing the substitution pattern. Commercial catalogues list it with product codes or supplier-specific shorthand, and database cross-referencing turns up entries in ChemSpider, PubChem, and regional chemical registers. Regulatory documents sometimes rely on legacy or trade names, so double-checking structural identifiers or spectral data remains wise, especially in highly regulated fields.
Safety & Operational Standards
Safe handling relies on straightforward habits. Ethoxyquinoline, like many aromatic heterocycles, requires gloves, goggles, and lab coats to avoid skin or eye contact. Risks reflect both acute toxicity and cumulative exposure, so fume hood use comes standard for weighing, transfer, and reaction procedures. Inhalation of vapors and dust poses hazards, based on analogs in its chemical class. SDS documentation spells out fire-fighting guidance—CO2, dry chemical, or foam—and spills need prompt cleanup with inert absorbents. Waste disposal aligns with local and national guidelines for organic chemicals, and storage protocols keep bottles cool, dry, and sealed away from incompatible agents like strong acids or oxidizers. Regulation-driven audits push process safety, aligning industrial users with OSHA standards or the European CLP Regulation, tracking everything from personnel training to incident reports.
Application Area
Ethoxyquinoline serves broad needs between research labs, manufacturing floors, and testing stations. In pharmaceuticals, it often acts as a building block for compounds targeting bacterial infections or central nervous system ailments, a nod to its quinoline ancestry. Agrochemicals draw on its chemical stability and tunable reactivity, weaving it into synthetic pathways for seed treatments or crop protection agents. Material scientists examine its incorporation in specialty polymers or as a photostable additive in coatings. Analytical chemists use it in standardization protocols, especially for developing new detection methods. Its track record in academic research reflects ongoing curiosity—bioactivity screens, mechanistic probes, and new synthetic routes all count on robust sample availability.
Research & Development
Continuous exploration surrounds ethoxyquinoline, especially in medicinal and synthetic chemistry. Researchers screen new derivatives for antimicrobial, antiviral, and anticancer potential, leveraging the structure for targeted activity and lower resistance profiles. Teams optimize synthetic routes, hunting for greener solvents or catalyst systems to cut down on waste or improve selectivity. Those working on analytical chemistry investigate stability, method validation, and trace impurity analysis, with practical feedback feeding back into product specifications. Collaboration between academia and industry keeps the research pipeline active, with published findings triggering commercial interest and, often, the next round of funding.
Toxicity Research
Evaluating safety means digging into detailed toxicological studies. Early animal data point to moderate acute toxicity, with liver and kidney functions in particular drawing scrutiny at high doses, echoing patterns seen in similar quinoline-type compounds. Chronic exposure studies remain ongoing, given concerns about possible mutagenic or carcinogenic effects, although definitive results often lag behind industrial demand. Regulatory agencies like the EPA and ECHA track hazard data and review all available findings before granting approval for new uses. In my experience, companies investing in significant production also carry out their own in-house toxicity screens, supplementing published data with case-specific tests under actual use conditions, especially in environments where end-product purity directly links to consumer safety.
Future Prospects
The next chapter for ethoxyquinoline opens up in two main directions. On one side, green chemistry drives the call for safer syntheses, with AI-assisted experimentation helping to pinpoint optimal conditions for minimal waste and energy use. On another, advances in bioinformatics and drug design continue to fuel medicinal investigations, as new analogs of ethoxyquinoline show promise against emerging threats like antibiotic-resistant bacteria and novel viral pathogens. Researchers also watch environmental fate and breakdown, aiming to balance industrial utility with responsible stewardship. As regulatory scrutiny intensifies, companies prepare for tougher disclosure and documentation, supported by real-world studies and transparent reporting. This compound’s journey reflects a broader trend in specialty chemicals—balancing performance, innovation, and safety with practical hands-on knowhow from the bench.
What is Ethoxyquinoline used for?
The Role of Ethoxyquinoline in Modern Industry
Ethoxyquinoline walks into conversations on chemical additives more often than most folks realize, especially where both agriculture and manufacturing overlap. It finds uses that go beyond just tweaking a formula; this compound actually tips the scale for shelf life in animal feeds and certain crop treatments. As someone who grew up around farm supply stores and still chats with old acquaintances working in poultry operations, I see first-hand how additives like this shape costs, health, and even the flavor of what ends up on dinner tables.
Championing Feed Preservation
Across the animal feed industry, spoiled product turns profit into loss in unspectacular fashion—silage that stinks, kibble that crumbles. Ethoxyquinoline performs as a preservative, mainly due to its antioxidant properties. Antioxidants stop fats and oils in feed from breaking down when exposed to air. This doesn't just save money; it ensures consistency for animals, especially in big poultry or fish operations. From my time working with aquaculture startups in the Pacific Northwest, I witnessed how feed stability keeps both the tanks cleaner and the fish growing on schedule. Less spoilage also means smaller farmers can depend on their bag of feed lasting through the entire training or breeding cycle.
Impact on Animal Health
Preservation isn't the end of the story. Oxidized fats not only smell bad—they can be downright dangerous to animal health, contributing to lower growth rates, immune system issues, or digestive problems. Adding proven antioxidants lowers these risks. Some papers published by the World’s Poultry Science Association have documented better overall flock performance whenever stable feed additives like ethoxyquinoline stay in the regular rotation. My own college research touched on this while I interned at a poultry nutrition lab, tracking mortality rates across batches given different feed formulations. The difference wasn’t only noticeable; it was measurable, even by undergraduate hands. Ensuring health on a broad scale means longer-lived animals and less waste, which ultimately filters down to better food safety standards for people as well.
Concerns Around Residues and Regulation
Questions always hover around chemical additives. Regulatory agencies in the US and Europe have debated how much ethoxyquinoline should show up in meat, eggs, or milk. Some markets set tough limits. Others call for full labeling. My time helping out on niche dairy farms hammered home just how cautious families can get about anything unfamiliar in their feed. Regulatory science, like that from the Joint FAO/WHO Expert Committee on Food Additives, highlights that low concentrations pose minimal health risk when used correctly. Fact-based transparency about dosage, potential buildup, and withdrawal times helps keep trust intact all the way from farm to fork.
Looking Forwards: Cleaner Agriculture
There’s momentum pushing for non-chemical ways to preserve feed—think vacuum-sealed storage or natural antioxidants like vitamin E. Yet in large-scale settings, the cost-to-benefit ratio of reliable synthetic antioxidants like ethoxyquinoline keeps them in headlines and supply rooms alike. Farmers want predictable results. Scientists push for ongoing studies. I’d argue that practical solutions rest on smart governance, real-time monitoring, and support for transparent education—making sure everyone along the chain knows both the benefits and the limits of what’s getting mixed in each batch. That keeps animal productivity up, protects people, and nurtures trust all at once.
Is Ethoxyquinoline safe for human consumption?
Big Claims, Big Questions
Food additives spark debates everywhere. Ethoxyquinoline is no exception. This synthetic compound shows up in preservatives aimed at making food last longer, and its use keeps drawing attention. Shoppers want to know if the product in their pantry actually supports their health goals or if it risks problems down the road. Nobody wants to unknowingly add a questionable chemical to their dinner plate.
Understanding the Debate
People don’t get the same information about additives like ethoxyquinoline as they do about calories or salt. Scientific names rarely end up in household conversations. Yet, what goes into food matters more than ever, especially as more people deal with allergies, chronic diseases, and the side effects of modern life. Regulatory bodies such as the FDA and European Food Safety Authority carry the responsibility of figuring out what’s safe enough to eat. They check chemicals for toxicity, interactions, and effects after long periods. This sounds simple, but research takes years, and new data can flip decisions on their head.
What the Science Says
Some studies raise red flags. High doses in animal trials have triggered concerns about possible toxicity and effects on the liver or thyroid. It’s a reminder that just because something preserves fish meal or pet food, it doesn’t mean it helps people thrive. On the flip side, regulators have set limits, arguing that trace levels likely don’t harm humans who aren’t overexposed. But the trouble comes from a lack of long-term data on daily human exposure. That leaves a grey area for anyone who wants clear-cut answers.
Real-World Risks and Transparency
Labels don’t always spell out what’s really inside a food item. Manufacturers might not use the same words consumers see in news stories. Ethoxyquinoline could appear under a code or blended within another ingredient. People relying on ready-made, processed foods take in small amounts of dozens of additives every day. No single serving seems like a risk, but for someone who eats these foods at nearly every meal, this steady intake can raise questions no one is yet fully able to answer.
The Role of Companies and Lawmakers
Food companies can choose to phase out additives that spark public concern, even if laws still permit them. This approach earned public trust in past cases, like with trans fats or artificial coloring in kids' snacks. Regulators benefit from honest, up-to-date research shared with the public in clear, direct language. The responsibility also falls on lawmakers to keep an eye on new studies and react quickly to evidence. Dragging out decisions or hiding data only deepens the trust deficit between the public and the food industry.
What Can Consumers Do?
Reading food labels closely, sticking to whole foods, and supporting brands committed to transparency help consumers limit their intake of unfamiliar additives. Anyone who’s ever managed a health scare in the family knows the daily choices add up. Staying curious—and a bit skeptical—usually serves health-conscious shoppers best. If questions remain about ethoxyquinoline, choosing food with the shortest ingredient list might just prove the safest call for now.
What are the side effects of Ethoxyquinoline?
A Closer Look at a Little-Known Compound
Ethoxyquinoline has turned up in conversations about food safety, animal feed additives, and chemical preservative use. Many folks don’t spend their afternoons thinking about obscure compounds, but the products we use every day sometimes carry more baggage than we realize. Being alert to the health risks linked to lesser-known chemicals can make a real difference in public health decisions.
What Happens After Exposure?
Direct information on ethoxyquinoline’s toxicology doesn’t flood the news cycle. In the world of chemical preservatives, research on human exposure often lags behind practical use. Labs have run animal studies that raise warning flags. Even small doses over a long stretch can affect the liver, kidneys, and skin. A study from the National Institute of Environmental Health Sciences reported how exposure in rodents changed liver enzymes and sometimes triggered inflammation or even cellular damage. The bottom line? The body doesn’t always process this compound smoothly, so it can pile up and stress organs over time.
People exposed to high levels sometimes notice mild symptoms first—mild headaches, gut discomfort, or skin irritation. Those with allergic tendencies might break out in rashes or hives. At higher exposures, the liver works harder to clean out toxins, which can show up in blood tests as changes in liver function. These early signs usually slip by unchecked in daily life unless someone knows what to look for. Doctors may not even connect the dots right away if the exposure isn’t obvious.
What Does the Science Say?
Regulatory groups in North America and Europe keep a close watch on chemicals used in food and feed, but loopholes exist. In the late 1990s, some fish farms faced pushback over the use of related chemicals as antioxidants in feed. Chronic exposure, especially in jobs where workers handle bulk preservatives, raises concerns about lung irritation, eyes watering, or chronic coughs. Animal studies suggest prolonged contact leads to tumors or reproductive harm, but the jury is still out on what that means for casual, low-level human use.
Community and Consumer Concerns
Stories from the field shape public opinion, sometimes faster than academic studies ever can. If you work in food processing or farming, you probably know someone who worries about what sneaks into the food supply. The risk often hits rural and working-class families harder. Worldwide, food safety scandals have pushed regular folks to demand more transparency about what goes into their meals.
Many consumers now look for ingredient lists on packages and press companies for safer preservatives. Public pressure works. Some food producers swap out riskier additives when faced with enough noise from customers and scientists. Advocacy for clearer labeling and regular independent safety reviews drives the industry forward, even when regulation lags.
Charting a Path Forward
Better research, stronger regulation, and a louder community voice set the stage for safer choices. Practical steps can help prevent side effects—regular testing of additives for both short- and long-term impacts, greater funding for toxicology studies, and policies ensuring that potentially risky chemicals don’t make their way into food or animal feed without proper vetting. Anyone who serves or produces food has a role to play in pushing for these changes. Using that collective voice builds trust and, ultimately, safer food for everyone.
How should Ethoxyquinoline be stored?
Paying Attention Keeps Everyone Safe
People working with chemicals often focus on big dangers, but small storage details can trip you up fast. Ethoxyquinoline doesn’t make headlines, yet storing it wrong creates more work and real safety risks. I remember watching a co-worker race to clean up a leaky chemical drum—he forgot to check the cap, and fumes spread through the whole storeroom. Even a standard product like Ethoxyquinoline demands respect.
Temperature Control Matters
A cool, dry place beats a steamy warehouse every time. High heat speeds up chemical breakdown and creates pressure that pushes open containers. Ethoxyquinoline prefers consistency—stashing it away from sunlight and heaters avoids ruined batches and keeps everyone breathing easier. OSHA and NIOSH stress that a temperature range below 25°C works best for storage, and avoiding direct sunlight preserves quality. Fluctuating or high temperatures helped cause more than one storeroom accident at places I’ve worked.
Keep It Dry and Airtight
Moisture never makes chemical storage better. Humidity in the air slips into bottles and drums, turning powders to clumps and liquids to unstable messes. With Ethoxyquinoline, a tightly sealed container blocks the worst problems before they start. Manufacturers use glass or high-quality plastic bottles for a reason—old, cracked containers invite trouble. Even taping over a weak spot rarely saves you from a cleanup later. It pays to triple-check lids and stoppers, especially if the product gets moved a lot.
Labeling Cuts Down on Costly Mistakes
Lousy labels waste money and create headaches. You might think everyone remembers every bottle after one day, but months later there’s no chance. I’ve sorted through unmarked bottles and wasted hours to test what’s inside. A simple label with product name, concentration, date, and hazard warnings keeps new hires and old hands on the same page. The CDC and most safety manuals insist clear identification prevents mix-ups and emergency calls. Quick double-checks in the stockroom stop big problems outside it.
Avoid Mixing and Bad Neighbors
Some chemicals just don’t play well together. I once saw bottles of acids stacked near solvents and, within days, the containers weakened and we had a near-miss. Separating Ethoxyquinoline from oxidizing agents, acids, and strong bases becomes non-negotiable. Cross-contamination destroys both safety and product value. Using designated shelves or full containment cabinets blocks chemical reactions. Safety protocols aren’t just red tape—they save real money and real lives.
Ventilation and Personal Protection Always Count
Strong odors may seem like a minor annoyance, but they signal a bigger issue. Good airflow in storage areas limits exposure. Most facilities rely on exhaust fans or even just open windows in well-built storage spaces, keeping fumes down. I’ve seen workers suffer headaches after ignoring this. Anyone handling Ethoxyquinoline benefits from gloves, goggles, and the occasional mask. No matter the product, protecting yourself and those around you never goes out of style.
Build Good Habits, Prevent Big Problems
Safe Ethoxyquinoline storage takes time and discipline. Stick with reliable habits: check labels, seal containers, control temperature and humidity, separate incompatible substances, and keep things ventilated. Trained staff and regular inspection keep every batch safer, fresher, and less likely to end up on a clean-up report. Sensible practices support the bottom line and keep people out of trouble—a lesson learned in every lab and warehouse.
Is Ethoxyquinoline approved by regulatory authorities?
What’s the Fuss About Ethoxyquinoline?
Ethoxyquinoline has caught the eye of scientists and industry insiders for its chemical properties. This compound, belonging to the quinoline family, has been considered for a few applications, like serving as an antioxidant in industrial or agricultural settings. Its name might not ring a bell for most, but for those of us keeping track of what goes into the food chain—or the products that reach our homes—a lot depends on which chemicals clear the regulatory hoops.
Regulatory Stance: Walking a Cautious Path
So, has Ethoxyquinoline won the green light from the major authorities? The story so far: neither the US Food and Drug Administration (FDA) nor the European Food Safety Authority (EFSA) have given their stamp of approval for its use in food, animal feed, or consumer goods. Search through regulatory databases, and you’ll find Ethoxyquinoline missing from lists of approved food additives or feed preservatives.
Safety testing forms the backbone of regulatory decisions. Agencies demand concrete evidence that any new chemical, especially those entering the food chain, poses minimal risk at real-world exposure levels. Without a thick stack of long-term safety studies, substances like Ethoxyquinoline end up in a gray area—neither outlawed nor cleared for wide use. In my years following regulatory news, this sort of limbo creates confusion for manufacturers and consumers alike.
Lessons from Similar Compounds
Learning from Ethoxyquinoline’s chemical relatives sheds some light. Take Ethoxyquin, another antioxidant once used in animal feed and pet food. Decades back, its approval came easily, but questions began to pile up about possible links to organ damage or cancer at high exposure. Countries like those in the EU started pulling approvals for Ethoxyquin, pushing companies to find safer options. With this backdrop, it’s no surprise regulators take their time with compounds that look or act in a similar way.
Consumers have grown wary of unfamiliar additives. Each time a news story surfaces about a chemical pulled from shelves or linked to health concerns, that trust weakens. Companies might want to introduce something new, yet regulators see the mess left by past mistakes and get even more cautious.
What Should Happen Next?
Clear, independent research sets the stage for any meaningful conversation with regulatory bodies. Toxicity studies, real-life exposure assessments, and environmental persistence all matter. Without them, regulatory agencies stay unconvinced. Some companies try to get ahead by running their own tests, but history shows that open access to data—outside the four walls of corporate labs—serves the public best.
Transparency in science and policymaking works both ways. Keeping stakeholders in the loop, especially public health experts and concerned citizens, creates more trust in whatever decision regulators reach. If Ethoxyquinoline ever gets a closer look, the push for comprehensive and public evidence will only grow stronger. Until then, it stands as another example of a compound stuck on the outside of official approval lists, waiting for more clarity about its safety.
Moving Toward Smarter Approval Process
Technology keeps evolving. High-throughput screening, advanced modeling, and new tools make it easier to assess chemical risks, cutting down the cost and time needed for regulatory review. A smarter, faster, and more open process means fewer unknowns slip through—or just as important, that useful innovations get a fair shake. Real progress means blending scientific rigor with the kind of transparency that everyone—from global corporations to backyard gardeners—can trust. Ethoxyquinoline’s status highlights the balancing act regulators perform: not just what gets in, but what keeps us safe.
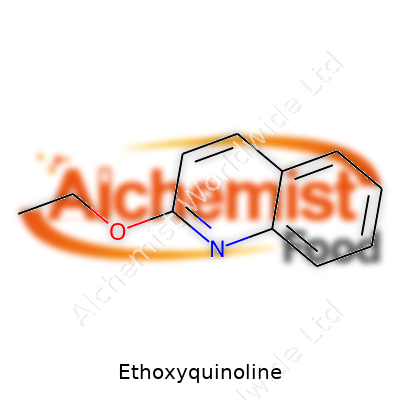
| Names | |
| Preferred IUPAC name | 2-ethoxyquinoline |
| Other names |
6-Ethoxyquinoline Quinoline, 6-ethoxy- |
| Pronunciation | /ɪˌθɒksiˈkwɪnoʊliːn/ |
| Preferred IUPAC name | 1-ethoxyquinoline |
| Other names |
Ethoxychinoline 6-Ethoxyquinoline |
| Pronunciation | /ɪˌθɒksɪkwɪnəˌliːn/ |
| Identifiers | |
| CAS Number | 578-74-5 |
| 3D model (JSmol) | `3D structure; JSmol; C11H11NO (Ethoxyquinoline)`: `LJVBQZQZBRPSFO-UHFFFAOYSA-N` |
| Beilstein Reference | 0132269 |
| ChEBI | CHEBI:140144 |
| ChEMBL | CHEMBL2106232 |
| ChemSpider | 21559654 |
| DrugBank | DB14097 |
| ECHA InfoCard | 17f1e734-50e2-45da-a743-3bffb4c733a2 |
| EC Number | 226-530-1 |
| Gmelin Reference | 2135954 |
| KEGG | C18622 |
| MeSH | D015242 |
| PubChem CID | 15611 |
| RTECS number | GV8575000 |
| UNII | R5V9VJ8Z8G |
| UN number | UN3077 |
| CAS Number | 147-63-9 |
| Beilstein Reference | 359368 |
| ChEBI | CHEBI:138495 |
| ChEMBL | CHEMBL2106624 |
| ChemSpider | 202798 |
| DrugBank | DB13523 |
| ECHA InfoCard | ECHA InfoCard: 100.041.800 |
| EC Number | 225-713-6 |
| Gmelin Reference | Gmelin Reference: 87294 |
| KEGG | C18622 |
| MeSH | D014960 |
| PubChem CID | 262361 |
| RTECS number | GO3140000 |
| UNII | 9A6B786HIM |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID7045139 |
| Properties | |
| Chemical formula | C11H11NO |
| Molar mass | 215.27 g/mol |
| Appearance | Yellow powder |
| Odor | Aromatic |
| Density | 1.1 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 2.9 |
| Vapor pressure | 0.000106 mmHg at 25°C |
| Acidity (pKa) | 5.20 |
| Basicity (pKb) | 4.90 |
| Magnetic susceptibility (χ) | -85.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.607 |
| Dipole moment | 3.73 D |
| Chemical formula | C11H11NO |
| Molar mass | 215.27 g/mol |
| Appearance | Yellowish crystalline powder |
| Odor | aromatic |
| Density | 1.1 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.7 |
| Vapor pressure | 0.000242 mmHg at 25°C |
| Acidity (pKa) | 5.20 |
| Basicity (pKb) | 6.17 |
| Magnetic susceptibility (χ) | -72.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.620 |
| Viscosity | 2.3 mPa·s (25 °C) |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -35.0 kJ/mol |
| Std molar entropy (S⦵298) | 387.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | Ethoxyquinoline: ΔfH⦵298 = 22.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4004 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| Precautionary statements | P280: Wear protective gloves/protective clothing/eye protection/face protection. |
| NFPA 704 (fire diamond) | 2-1-1 |
| Flash point | Flash point: 113°C |
| Autoignition temperature | 385°C |
| Lethal dose or concentration | LD50 (oral, rat): 370 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Ethoxyquinoline: 620 mg/kg (oral, rat) |
| NIOSH | JP9625000 |
| PEL (Permissible) | PEL for Ethoxyquinoline is not specifically established by OSHA. |
| REL (Recommended) | 0.05 mg/kg bw |
| IDLH (Immediate danger) | Not established |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H312, H332, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P301+P312, P302+P352, P305+P351+P338, P308+P313, P330, P332+P313, P337+P313, P362+P364, P501 |
| Flash point | 113°C |
| Autoignition temperature | 470°C |
| Lethal dose or concentration | LD50 (rat, oral): 600 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Ethoxyquinoline: 620 mg/kg (rat, oral) |
| NIOSH | JP3575000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 30 mg/L |
| Related compounds | |
| Related compounds |
8-Hydroxyquinoline Quinoline Ethoxyquinoline sulfate Ethoxyquinoline hydrochloride |
| Related compounds |
Quinoline 2-Ethoxyquinoline 4-Ethoxyquinoline 6-Ethoxyquinoline 8-Ethoxyquinoline Ethoxyquinoline-3-carboxylic acid Chloroquinoline Methoxyquinoline Hydroxyquinoline |

