DL-Malic Acid: A Modern Perspective on an Old Chemical Ally
Historical Development
DL-Malic acid made its debut through the careful gaze of early chemists exploring natural acids. Carl Wilhelm Scheele first isolated malic acid from apples in the late eighteenth century, though at that time, the exact stereochemistry remained a mystery. As organic chemistry matured, malic acid's structure became clearer, revealing that both natural (L-form) and synthetic (DL-form) versions tell their own stories in various sectors. The commercial push for DL-malic acid kicked into gear as food processing looked for acids that would not only adjust flavor, but also add longevity to products lining grocery shelves.
Product Overview
With DL-malic acid, companies find a white crystalline powder that carries a sharp but not overwhelming sour taste. This acid punches up fruitiness in beverages, counteracts bitterness, and extends the shelf life of processed goods. Its versatility is hard to ignore. Not only does it work well in acidic candies, acidic drinks, and bakery fillings, DL-malic acid has gained ground in agriculture, animal feed, and cosmetics by acting as a buffer, pH adjuster, and mild exfoliant.
Physical & Chemical Properties
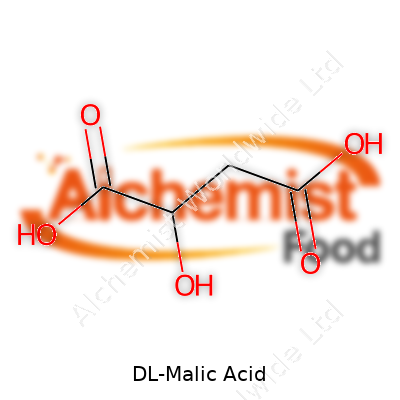
DL-Malic acid shows up as a solid white powder, easily dissolving in water and alcohol. Chemically named 2-hydroxybutanedioic acid, its molecular formula is C4H6O5. The melting point usually sits around 130ºC to 135ºC. This racemic mixture, made up of both D- and L-isomers, shares similar tartness to citric acid but does not match its intense acidity, so it leaves a rounder finish in foods. The acid’s two carboxyl groups bring flexibility for buffering and complexation in industrial settings.
Technical Specifications & Labeling
Producers offer DL-malic acid to meet specifications set by organizations like the Food Chemicals Codex, European Pharmacopoeia, and the United States Pharmacopeia. Purity levels usually top 99%, with moisture limits often below 2%. Users watch for lead and heavy metal content, as strict rules from health regulators drive these below 2 ppm. Labels must declare whether the product is the racemic blend, and food-grade batches require batch numbers, production dates, and country of origin. The safety sheet lists possible hazards, as well as proper storage and handling instructions, matching requirements seen in industrial workplaces and food manufacturing plants.
Preparation Method
Industry teams create DL-malic acid by hydrating maleic anhydride, a substance purchased in bulk for its reactivity. The hydration process runs at elevated temperatures and pressures, using catalysts like strong acids to speed things along. The result is a racemic mix, with both D- and L-forms produced in equal measure—ideal for industrial use where the natural isomer is less critical. Once produced, the acid is crystallized and then dried under controlled conditions to ensure a consistent, food-safe product that stands up to global testing standards.
Chemical Reactions & Modifications
DL-Malic acid steps up as a reagent in plenty of classroom and lab reactions. Its structure invites dehydration, oxidation, and reduction. Lab technicians use it to synthesize fumaric acid through dehydration. They use strong acids and heat to drive off water, leaving the trans-alkene. Chemists take advantage of its two carboxyl groups for esterification, turning it into diesters that play parts in perfumes, polyester resins, and pharmaceutical compounds. Oxidation with strong agents breaks it down to oxaloacetate, tying it into important metabolic cycles for biochemistry research.
Synonyms & Product Names
People have called DL-malic acid by several names across industries. Chemists might jot down 2-hydroxy-succinic acid, while the food sector goes with INS 296 or E296. Pharmacies and nutrition supplement labels cite Hydroxybutanedioic acid. Older textbooks and patent files mention its systematic IUPAC names, but on ingredient lists, you almost always see “malic acid” with or without a note about its form.
Safety & Operational Standards
Handling DL-malic acid does not pose severe hazards, but good workplace habits still matter. Dust inhalation may lead to mild respiratory irritation, so most factories use local exhaust ventilation and recommend dust masks. The acid is not as corrosive as stronger acids, yet skin and eye contact ought to be avoided just as a precaution. Food-grade material always passes toxicological screening and complies with international limits, underlining the priority for consumer safety. Storing the chemical in cool, dry areas, shielded from heat and moisture, keeps it free from clumping and preserves quality from production floor to warehouse.
Application Area
Food manufacturers lean heavily on DL-malic acid for its gentle tang, which helps offset sweetness in juices, soft drinks, and candies. It pops up in wine and spirit production to tweak flavor profiles and improve stability during aging. Cosmetic brands have woven it into facial cleansers and exfoliants, capitalizing on its ability to gently slough off dead cells. Agriculture finds use for it as a chelating agent and pH stabilizer in special fertilizers and feed mixes. Pharmaceutical makers look for its acidity to balance tablet formulations and make active ingredients more soluble. In each case, the chemical flexibility and cost-effectiveness appeal to those managing complex supply chains.
Research & Development
Recent R&D efforts examine greener synthesis, moving from petrochemical starting materials to biobased routes. Labs test fermentation processes using genetically engineered microbes, aiming to reduce carbon footprints and dependence on fossil fuels. Scientists peer into malic acid’s functions in metabolic engineering, working to adjust its production in yeasts and bacteria for both food and biofuel. Researchers also track how DL-malic acid partners with other acids to improve flavors in new plant-based foods and alternative protein drinks. In the field of materials science, its chemical backbone sparks ideas for new polymers and resins.
Toxicity Research
Toxicologists find DL-malic acid to have low toxicity across animal studies; typical dietary amounts do not cause harm. The Joint FAO/WHO Expert Committee on Food Additives recognizes malic acid as safe for consumption, noting that the body metabolizes malic acid through familiar Krebs cycle pathways. Stomach upset can occur if ingested in large quantities, but this holds for most acids and is not unique to malic acid. Regulatory agencies require thorough chronic exposure studies before allowing its widespread use in foods, and so far, evidence keeps pointing toward safety within the advised limits.
Future Prospects
DL-Malic acid faces an interesting road ahead as both synthetic methods and application areas keep shifting. Rising trends in plant-based eating and sugar reduction give DL-malic acid new ground to cover in creating authentic flavors and providing functional acidity. Sustainability pressures in chemical manufacturing continue to motivate greener production, moving from petroleum-based compounds to fermentation and renewable processes. In technical fields, its special chemical reactivity opens doors for smart materials, biodegradable plastics, and novel pharmaceuticals. Regulators keep raising the bar for safety and environmental health, challenging manufacturers to push transparency and purity even further. If one reality defines the outlook, it’s the steady demand for reliable, adaptable organic acids across industries navigating a fast-changing marketplace.
What is DL-Malic Acid used for?
Bringing Tartness to Foods and Drinks
DL-Malic acid gives many of our favorite foods their punch. That sharp, sour taste in a green apple candy? Often, DL-Malic acid is the secret. Soft drinks, sherbets, jams, some dairy treats—many of them rely on this sour flavor to wake up your taste buds. Winemakers use DL-Malic acid to tweak acidity; it smooths out bitterness and brings balance to the bottle. Bakers mix it into dough for a lighter, tangier result. If a product claims “artificial flavor,” there’s a fair chance DL-Malic acid delivers some of it.
Helping Supplements and Medicine Go Down Easier
Pharmaceutical companies use DL-Malic acid for more than just taste. Malic acid levels fall in some health problems, so supplements include this ingredient for muscle support and energy. It's a component in gummies, chewables, and liquid syrups to make them a lot easier to swallow. Some dental products use it because it stimulates saliva flow, helpful for folks battling dry mouth. The ingredient helps mask bitterness in vitamins and supports stable formulas, so brands can offer longer shelf life.
Shaping the World of Animal Feed and Agriculture
Animals, just like humans, crave palatable food. DL-Malic acid brings that appeal to livestock pellets and pet chow. With its sour kick, it makes feed more tempting, especially for young animals. On farms, it helps regulate the pH of silage and forage, supporting fermentation and cutting spoilage. Healthier feed translates into better growth, and in my time visiting family farms, good feed can make all the difference. Farmers watch these little tricks to save costs and raise healthier animals.
Boosting Cleaners and Industrial Products
The cleaning aisle may seem worlds away from snack shelves, yet DL-Malic acid works in both. Many bathroom or kitchen cleaners harness its power to cut through mineral stains and scale. Its acidity helps dissolve grime in hard water areas without harsh chemicals. It pops up in hair and skin products too—DL-Malic acid can exfoliate gently and adjust a product’s pH, keeping your scalp or skin feeling fresh.
Looking at Safety, Sourcing, and Environmental Impact
DL-Malic acid usually comes from chemical synthesis, combining maleic anhydride with water. Some producers extract it from fermented fruits or plants, offering a “natural” label. Either way, safety keeps popping up in my conversations with food makers. At practical levels, DL-Malic acid is generally recognized as safe in foods and supplements. It’s passed plenty of checks by the FDA and EFSA, but overdoing it can upset stomachs, especially for little kids or pets. Watching serving sizes makes a real difference.
Sustainability is another issue people care about. Mass production relies on petrochemical sources, raising questions about carbon footprints. Some new companies look to green chemistry and fermentation to cut these concerns. Shoppers and manufacturers can nudge the industry towards cleaner processes by choosing brands with transparent sourcing.
Possible Improvements and Better Decisions
Natural souring alternatives, like citric acid or tartaric acid, compete alongside DL-Malic acid. They don’t always deliver the same flavor or performance, but for simpler ingredient lists, they offer an easy swap. For more transparency, clearer labeling on packaging would help those who want to avoid synthetic additives or are sensitive to acids.
Investing in research for more eco-friendly production could lift DL-Malic acid’s standing across different markets. With better public understanding, people can pick products that fit their values, whether they're aiming for a cleaner process, greener chemistry, or just a tastier candy.
Is DL-Malic Acid safe for consumption?
Looking Beyond the Label
DL-Malic acid turns up on ingredient lists for everything from candies to sodas. The name might spook the average grocery shopper, especially since “acid” rarely sounds appetizing. So, what does it do, and more importantly, is it safe?
Understanding the Source
Malic acid is a natural component in fruits. Apples, for example, rely on L-malic acid for their signature tart kick. The “DL” in DL-malic acid stands for the combined D and L forms, with the L type being the same form found in those apples. The D form isn’t found in nature, but both forms can do the same job in foods. In the food industry, DL-malic acid adjusts pH, gives that desired tangy punch, and helps preserve freshness in products. Even if you never buy the pure powder, it sneaks into more items than most people realize.
Backed by Science
Governments and food safety experts dig into every additive before it gets a green light. The US Food and Drug Administration lists malic acid (including the DL form) as “generally recognized as safe” (GRAS). The European Food Safety Authority holds the same view, giving it the E296 number. These approvals don’t come lightly. Scientists run animal tests, look into possible health risks, and examine safe intake levels. Most people would have to eat extreme quantities to reach even close to concerning levels.
No Shortage of Studies
With a bit of research, you’ll find studies that cover how malic acid works in the human body. It enters natural metabolic pathways. Our bodies know what to do with it and break it down just like the malic acid in fruit. Reports show people eating foods with small amounts of malic acid for years without any public health warnings tied to the ingredient. Allergic responses are rare, and problems usually come from consuming far more than the typical diet provides.
Addressing the “Synthetic” Factor
Some folks worry about synthetic ingredients, and DL-malic acid often comes from laboratory production. The reality is, our bodies can’t tell the difference between the natural and synthetic forms—they get absorbed and metabolized the same way. The same principle applies to vitamins or sweeteners we see in multivitamins or sugar packets. Plenty of safe, beneficial ingredients start in the lab. What matters is the rigorous safety process each undergoes before it lands in your snack or drink.
Why Transparency Matters
People should know what they’re putting into their bodies. Food companies and regulators publish ingredient lists for a reason. Easy access to food science helps everyone make smart choices for themselves and their families. If someone has worries about acidity, certain digestive conditions, or specific intolerances, then chatting with a dietitian or medical professional makes sense. For most people, moderation solves the puzzle. DL-malic acid, found almost everywhere in processed foods, serves as a safe, practical ingredient when used correctly. Knowledge brings confidence at the grocery store and in the kitchen.
What a Balanced Diet Looks Like
It always helps to keep things simple—fruits, veggies, grains, proteins. That doesn’t mean all ingredients with complex names should set off alarms. With malic acid, decades of safe use, strong research, and transparent labeling show the ingredient earns its spot on ingredient lists. If you want to limit processed foods, that’s a personal choice. Just know that DL-malic acid on a label signals a trusted tartness, not a hidden danger.
What is the difference between DL-Malic Acid and L-Malic Acid?
Understanding the Science in Simple Terms
Staring at a supplement label or food ingredient list, two nearly identical names pop up: DL-malic acid and L-malic acid. They look similar, but those two letters make a difference. L-malic acid turns up in apples, cherries, and actually in every human body. DL-malic acid contains both the “L” version and its mirror, called “D.” Imagine shaking hands with your right and left hand—same outline, but not the same fit.
How the Body Actually Handles Each One
L-malic acid forms naturally during the cycle that lets us turn food into energy. Our muscles, brain, and even skin rely on the L-form. This natural acid ends up in sour candies, yogurts, and sports drinks because it brings a bright, tart flavor and helps with energy pathways. Food scientists trust it, backed up by decades of research.
DL-malic acid carries both the natural L- form and the synthetic D-form. The body recognizes and uses the L-form without trouble. The D-form, though, doesn’t play a role in human energy cycles. Our systems can’t use it the same way, and some research shows that too much of the D-form can add unnecessary baggage for the kidneys to filter. It’s not about toxicity at realistic food levels, but efficiency. That’s the real divide: the L-form matches what our body expects; the D-form is more like an extra passenger.
Food Industry Choices, Nutritional Impacts
L-malic acid in pure form makes sense for sports nutrition and wellness, especially if someone has kidney challenges or wants to follow the body’s own blueprint. Athletes and active folks sometimes look for L-malic acid as a safe, efficient player in muscle recovery and stamina.
DL-malic acid drops cost for food manufacturers since making both forms in the lab is faster and cheaper than extracting just the L-form from fruit. Producers still use it for the same zing and flavor, so most people would never notice the swap unless carefully checking ingredients. Some chewing gums, supplements, and processed foods lean toward the DL-mix for budget reasons.
Label Reading Matters
People with specific health needs, especially individuals with kidney trouble or rare enzyme deficiencies, should look for L-malic acid over DL-malic acid. For everyday eaters, both serve as flavor agents, but those curious about the cleanest fit for biochemistry often choose the L-form.
Trust builds from transparency. Top food brands and supplement companies aim for purity—not just to look good, but because informed consumers demand it. In recent years, some leading sports drink brands and clean-label supplement makers have moved toward using pure L-malic acid, investing in consumer education instead of only cost savings. This shift stands as a signal: clarity helps everyone, even if it requires spending a few more cents per serving.
Looking Ahead
Consumers who care about the contents of their snacks and supplements can support better choices by reading labels, asking questions, and demanding clarity from manufacturers. Industry responses tend to follow consumer voices—clear demand for pure, research-backed ingredients creates real change. By choosing L-malic acid, people can align their nutrition choices with how nature and the human body work best.
What are the common applications of DL-Malic Acid in food and beverages?
Making Flavors Pop in Soft Drinks and Juices
DL-Malic acid lands on the ingredient list for a good reason—it packs a tart punch that can turn a flat-tasting lemonade into something bold and tongue-tingling. A splash of malic acid in apple juice adds brightness, making the flavor more crisp and less sugary sweet. If you scan the label on sour candies or energy drinks, you’ll see it listed alongside citric acid. That’s no accident. Malic acid layers in extra tartness, giving candies and drinks a longer-lasting sour kick without going overboard with sugar.
Keeping Foods Fresh and Stable
Sour flavor isn’t the only skill malic acid brings to the kitchen. This ingredient also stabilizes pH, which helps certain foods last longer on the shelf. Fruit pies, jams, and jellies depend on consistency and color. With the right acid balance, home-canned peaches won’t turn brown, and that supermarket cherry pie looks as fresh as the day it went in the oven. Bakers know it’s easy to lose tartness or freshness after just a few days, so a bit of malic acid in fillings and doughs helps lock in a just-picked taste. It even works in syrups and preserves, where it keeps products appealing during transport or storage without resorting to heavy-duty preservatives.
Supporting the Right Rise in Baked Goods
Bakery chefs run into a classic challenge—getting the right lift in cakes or muffins. Malic acid comes into play where baking needs a push. Blended with leavening agents like baking soda, it triggers a reaction that creates just the right amount of carbon dioxide bubble action. This helps products gain a moist, airy texture instead of feeling dense or crumbly. The bread stays soft, and the texture doesn’t go stale after sitting on the table for a day.
Balancing the Sweetness in Reduced-Sugar Foods
People want to cut back on sugar but keep the taste they love. DL-Malic acid acts as a flavor booster for sugar substitutes, which can sometimes taste flat or chemical. It sharpens flavors in diet sodas, light jams, and low-calorie candies. A 2012 study published in the Journal of Food Science highlighted how malic acid creates a more balanced, full taste in sugar-reduced products, with tasters reporting a fruitier, rounder flavor profile compared to batches made with citric acid alone.
Shaping the Future: Safer, Tastier Choices
With more people caring about clean labels and fewer artificial chemicals, ingredient choices matter. DL-Malic acid, produced through fermentation or chemical synthesis, passes through safety evaluations by groups like the U.S. Food and Drug Administration. Still, if manufacturers highlight its plant-based origins and focus on sustainable sourcing, customers could feel even better about picking up that bottle of juice or pack of sour gummies. The push for transparency also means keeping tabs on usage levels to avoid overpowering flavors or causing digestive discomfort. It’s a balancing act, and experienced food technologists often consult peer-reviewed research to set industry standards.
Pushing for Healthier Recipes
In decades working with nutritionists and chefs, I’ve seen how simple swaps make a difference. Adding malic acid allowed many brands to cut back on sodium benzoate or ascorbic acid as sole preservatives. For those sensitive to specific additives, this opens doors to snacks and drinks that taste as fresh as the real thing. As more people read ingredient lists, smart use of DL-malic acid helps deliver both flavor and shelf life—without a pile of numbers and chemical names on the label.
Are there any side effects associated with DL-Malic Acid?
Understanding DL-Malic Acid
DL-Malic acid pops up in foods, drinks, and even supplements. The tang in green apples traces straight to this compound. Sometimes, manufacturers add it to boost flavor or tweak acidity. In other cases, athletes or nutrition advocates explore it for an energy lift or muscle recovery. All these uses might sound harmless, but risks start to show up when people add extra malic acid to their routine, especially outside food sources.
Common Side Effects at a Glance
Those who stick to amounts in food rarely report trouble. The gut handles small supplies of DL-malic acid just fine. The story can change after loading up with supplements or excessive fortified products. The most common complaints involve sore stomach, diarrhea, or a churning gut. Large doses mess with the digestive lining, cranking up acid where the body doesn't want it. People often think more is better, yet that approach rarely lands well with acids of any kind.
Who Should Watch Their Intake?
A healthy adult might tolerate some extra DL-malic acid, but anyone with chronic digestive issues faces more risk. People with acid reflux, ulcers, or irritable bowel syndrome sometimes react harshly. Even folks without big digestive problems can struggle with heartburn or general soreness after overdoing it. Kids and older adults also get hit harder by additives like these, so parents need to keep a close eye on how much turns up in snacks or drinks.
Scientific Perspective and Real Experiences
Research points out incidents where supplements cause stomach pain and diarrhea. A paper published in the Journal of Dietary Supplements outlined several case studies involving high malic acid intake, all showing gastrointestinal symptoms within hours. My own experience matches the science—I tried a performance supplement that listed DL-malic acid as a main ingredient, and within a day felt cramping and discomfort I couldn’t chalk up to anything else. Conversations with dietitians add weight here: most agree the acid’s safe in food amounts, but untested supplement blends crank up the risk without offering guarantees for safety.
Less Common Concerns
Allergic reactions stand as rare, but not impossible. Someone sensitive to similar compounds could develop swelling, itching, or rashes. Headaches and mild dizziness pop up in reports, too, though studies link those mostly to huge doses. Misuse can tip body chemistry in uncomfortable directions, especially when taken without a meal or with other acidic supplements. People with kidney disorders especially should skip DL-malic acid supplements, since extra acid might burden the kidneys.
Finding Healthy Balance
Food and beverage labels list DL-malic acid under "additives" or "acidity regulators." Staying aware of daily consumption makes a difference, especially for families using many packaged foods. Most folks don’t need to hunt it down for health benefits, as a good diet covers basic needs. If a supplement seems necessary, consulting a doctor or registered dietitian takes top priority—anyone can have a unique reaction, and seeing the full health picture matters. For folks who crave energy boosts or improved exercise recovery, safer options exist. Enough sleep, steady hydration, and fresh whole foods offer more support than any powder or pill with unknown risks.
| Names | |
| Preferred IUPAC name | 2-hydroxybutanedioic acid |
| Other names |
DL-2-Hydroxysuccinic acid DL-Hydroxybutanedioic acid 2-Hydroxybutanedioic acid DL-Apple acid |
| Pronunciation | /diː-ɛl ˈmælɪk ˈæsɪd/ |
| Preferred IUPAC name | 2-hydroxybutanedioic acid |
| Other names |
DL-Applesäure DL-2-Hydroxysuccinic acid DL-MAE DL-2-Hydroxybutanedioic acid |
| Pronunciation | /diː-ɛl ˈmælɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 617-48-1 |
| 3D model (JSmol) | `load =3d4e` |
| Beilstein Reference | 82163 |
| ChEBI | CHEBI:32453 |
| ChEMBL | CHEMBL1406 |
| ChemSpider | 566 |
| DrugBank | DB04248 |
| ECHA InfoCard | ECHA InfoCard: 100.003.277 |
| EC Number | EC 200-711-8 |
| Gmelin Reference | 1522 |
| KEGG | C01807 |
| MeSH | D001320 |
| PubChem CID | 525 |
| RTECS number | OO5250000 |
| UNII | F0Y76J35Q7 |
| UN number | UN3077 |
| CAS Number | 617-48-1 |
| Beilstein Reference | 1720231 |
| ChEBI | CHEBI:10607 |
| ChEMBL | CHEMBL1233314 |
| ChemSpider | 14414 |
| DrugBank | DB04272 |
| ECHA InfoCard | ECHA InfoCard: 100.003.287 |
| EC Number | EC 200-293-7 |
| Gmelin Reference | 8416 |
| KEGG | C00852 |
| MeSH | Dicarboxylic Acids |
| PubChem CID | 525 |
| RTECS number | OO5250000 |
| UNII | J94594Y9U7 |
| UN number | UN1760 |
| CompTox Dashboard (EPA) | EPA CompTox Dashboard for DL-Malic Acid: **DTXSID5046487** |
| Properties | |
| Chemical formula | C4H6O5 |
| Molar mass | 134.09 g/mol |
| Appearance | white crystalline powder |
| Odor | Odorless |
| Density | 1.601 g/cm³ |
| Solubility in water | Fully soluble in water |
| log P | -1.26 |
| Vapor pressure | 0.0064 hPa (25°C) |
| Acidity (pKa) | 3.40, 5.11 |
| Basicity (pKb) | 1.92 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.585 |
| Dipole moment | 3.59 D |
| Chemical formula | C4H6O5 |
| Molar mass | 134.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.609 g/cm³ |
| Solubility in water | Miscible |
| log P | -1.26 |
| Vapor pressure | <0.1 hPa (20°C) |
| Acidity (pKa) | 3.40, 5.11 |
| Basicity (pKb) | 12.45 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.585 |
| Dipole moment | 1.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 151.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -955.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1348 kJ/mol |
| Std molar entropy (S⦵298) | 154.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -886.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1345 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A16AX |
| ATC code | A16AA06 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation. |
| GHS labelling | GHS07, GHS eye irritation 2A, H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | NFPA 704: 1-1-0 |
| Autoignition temperature | 220°C |
| Lethal dose or concentration | LD50 (oral, rat): 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1600 mg/kg |
| NIOSH | WZ0450000 |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 2000 mg |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS hazard statements: H319 |
| Pictograms | GHS07, GHS05 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 214°C |
| Autoignition temperature | 220°C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (Rat, oral): 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 3200 mg/kg |
| NIOSH | SN4025000 |
| PEL (Permissible) | 30 mg/m³ |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
L-Malic acid D-Malic acid Succinic acid Fumaric acid Tartaric acid Citric acid |
| Related compounds |
Maleic acid Fumaric acid Succinic acid Tartaric acid Citric acid |

