DL-Ascorbic Acid: A Deep Dive into Its Development, Uses, and Impact
Historical Development
Long before vitamin C tablets showed up at pharmacy counters, scientists were on the hunt for something to tackle scurvy and boost immune systems. The story of ascorbic acid echoes the progress of modern chemistry as researchers like Albert Szent-Györgyi in the 1930s isolated ascorbic acid from food and gave it the spotlight in nutritional science. Over time, people in labs figured out ways to make both L-ascorbic and DL-ascorbic forms, the latter showing up as a synthetic creation. DL-ascorbic acid stands as a racemic mixture, offering both right- and left-handed molecular forms. While the natural L-form takes center stage in nutrition, the synthetic DL version didn’t go unnoticed, especially among manufacturers looking for alternatives in bulk production and specialized industrial needs.
Product Overview
DL-Ascorbic acid shares a close skeletal structure with natural vitamin C, though the difference in orientation changes how it works in the body. Producers issue this powdery substance as a white or slightly yellow solid, often shipped in sealed containers to retain its potency. In supplement and ingredient form, the product makes its way into sectors ranging from food preservation to fertilizer enhancement, often prized for its antioxidant qualities that can help stabilize blends or slow down spoilage in products sitting on the shelf.
Physical & Chemical Properties
On the lab bench, DL-ascorbic acid stands out for its crystalline texture, water solubility, and faint tangy scent reminiscent of citrus. It melts at about 192°C, forming a clear, slightly viscous liquid right before decomposition. Chemists note its molecular formula, C6H8O6, and pay attention to the fact that, due to its two chiral centers, DL-ascorbic acid contains both stereoisomers in equal measure. It readily dissolves in water and to a degree in alcohol, lending itself to a wide variety of formulations and blends. The real draw is its strong reducing power: it gives up electrons easily, which is what lets it snuff out free radicals or react in manufacturing processes.
Technical Specifications & Labeling
Suppliers of DL-ascorbic acid work hard to keep the product free of contaminants. Typical labeling puts the purity at above 99%, as determined by high-performance liquid chromatography or titration. Moisture content often sits beneath 0.5%, and particle size remains tightly controlled, which comes in handy for anyone measuring out precise doses or adding the powder to mixtures. This compound should be labeled by its CAS number (50-81-7 for ascorbic acid in general), and packages carry information on shelf life, recommended storage conditions—dry, away from light and heat—and hazard symbols according to GHS guidelines if sold in bulk.
Preparation Method
Manufacturers produce DL-ascorbic acid typically by chemical synthesis, starting with glucose as the feedstock. A series of oxidation and chemical rearrangement reactions, often relying on catalytic agents and temperature-controlled reactors, give rise first to L-sorbose and then to ascorbic acid. For the DL form, the synthetic route involves steps where both mirror-image forms result, skipping the orientation-specific biological enzymes that natural organisms use. The process taps into intermediates like diketogulonic acid and then cyclizes the molecule under controlled pH to get the final product. Advanced factories employ continuous feed, rigorous filtering, and drying systems to crank out uniform batches ready for shipment.
Chemical Reactions & Modifications
As a reducing agent, DL-ascorbic acid reacts swiftly with oxidizers, and this behavior grounds much of its use as a preservative or stabilizer in various products. Researchers and product developers modify the molecule by esterification to make fat-soluble derivatives, or by attaching other groups for improved stability or compatibility with non-polar mixtures. In industry, these transformations push ascorbic acid’s boundaries, letting it play a role in cosmetic emulsions, animal feed, or medical applications beyond traditional vitamin C use. Chemists keep close tabs on reaction conditions, since changes in pH and temperature can nudge the product toward unwanted side products or loss of activity.
Synonyms & Product Names
Across different markets and scientific references, DL-ascorbic acid pops up under several names: 3-Oxo-L-gulofuranolactone, racemic ascorbic acid, or just plain synthetic vitamin C. Ingredient lists in food and supplements might refer to it by E number (E300), and industrial supply catalogs describe it by code numbers or trade names that blend ascorbic acid with brand labeling. This diversity of names often confuses consumers and buyers, so responsible suppliers include chemical identifiers and full IUPAC nomenclature for clarity.
Safety & Operational Standards
On the safety front, the focus lands on dust control, exposure limits, and personal protective equipment. DL-ascorbic acid typically exhibits low acute toxicity, but like any fine powder, it can irritate airways if inhaled. Workers handling sacks of the product use gloves, goggles, and dust masks, and guidelines call for storage in cool, dry, ventilated spaces to avoid clumping or degradation. Local and global regulations spell out blending tolerances for food and supplement use, while food safety authorities such as the FDA and EFSA lay down daily intake thresholds. Factories log batch numbers and test results to support traceability down the line in case issues pop up.
Application Area
Food processors make wide use of DL-ascorbic acid for its preservative and color-stabilizing effects, especially in sliced fruits, cured meats, canned goods, and baked products. Feed manufacturers add it to animal feed for shelf life extension and nutritional fortification. Chemical and pharmaceutical industries look to its reducing action to keep ingredients from breaking down during production or storage. There’s even use in water treatment and agriculture, where the compound helps buffer oxidative stress in plants or acts as a mild sterilizing agent. Despite its versatility, the nutritional community acknowledges that, for direct human supplementation, L-ascorbic acid alone supports bodily needs, and uses for the DL form mostly cater to industrial, cosmetic, or specialty chemical applications.
Research & Development
Research continues to probe the boundaries of DL-ascorbic acid. Studies focus on new synthesis routes with greener reagents or lower energy footprints. Some teams look to encapsulate or combine ascorbic acid with carriers that slow oxidization in food or skincare. There’s also work underway to tweak the molecule for targeted drug delivery or specialized chemical reactions. Patented methods now aim to separate the DL mixture into its L and D forms, hoping to create purer, value-added products for health-conscious markets or new therapeutic strategies. Investigation persists around the use of the D-form, less active in human systems, yet potentially beneficial in other biological or chemical roles.
Toxicity Research
Extensive testing on DL-ascorbic acid supports its general recognition as safe in limited quantities for food and industrial use. Researchers subject it to acute and chronic toxicity studies in animals, tracking metabolic breakdown and elimination rates. Evidence to date suggests low overall risk, though some byproducts formed under heat or extreme pH invite caution for certain formulations. Regulatory bodies review these findings before approving marketing or recommending exposure limits. Long-term consumption in doses exceeding dietary needs offers no benefit, so most guidance centers on moderation and specific, well-documented uses.
Future Prospects
The future for DL-ascorbic acid ties into broader questions about sustainability, innovative chemistry, and personalized nutrition. More companies show interest in scaling greener methods to produce ascorbic acid, aiming to lower energy use or shrink environmental footprints. Biotechnological advances open doors for custom-tailored derivatives with properties fit for next-generation foods, pharmaceuticals, or material sciences. Researchers keep looking for new structures and applications, sometimes inspired by the natural resilience that vitamin C brings to living systems. Even as the main story in nutrition sticks to the L-form, interest in the broader suite of ascorbic acid variants continues to fuel patent filings and basic science projects across university labs and industry think tanks.
What is DL-Ascorbic Acid used for?
What Is DL-Ascorbic Acid?
Most folks know about vitamin C, that tangy powder you might scoop into a morning smoothie or see stamped on the side of your orange juice carton. The story behind DL-ascorbic acid runs pretty close, though the chemistry books show an interesting twist: DL-ascorbic acid is a mix of two mirror-image forms of ascorbic acid, not just the well-known one found in food sources.
Why People Turn to DL-Ascorbic Acid
From the supplement aisle to the factory floor, DL-ascorbic acid shows up in more places than most realize. It serves as a preservative in food production. Think about opening a snack or canned fruit and expecting it to taste the same every time—DL-ascorbic acid helps keep color and flavor from fading by slowing down reactions that oxygen triggers. Families might not notice each time they eat lunch meat or juice that ascorbic acid stopped it from turning brown, but this compound shows real value in kitchens and warehouses alike.
Then there’s the supplement market. Nutrition brands often rely on synthetic vitamin C, which includes DL-ascorbic acid in some cases. While only the L-form joins in the body’s chemistry, the DL version offers a cost-effective, stable option for manufacturers. Some label-savvy shoppers steer clear of the “DL” blend, aiming instead for pure L-ascorbic acid. For large-scale operations, though, the DL mixture answers the demand for shelf-stable ingredients at a lower price.
How It Shows Up Outside the Kitchen
Beyond food, the pharmaceutical world sees potential in DL-ascorbic acid. Researchers investigate both antioxidant properties and how this compound interacts with other ingredients in blends. Some skin-care companies experiment with ascorbic acid’s ability to brighten complexion and support healthy-looking skin. Using the right form becomes crucial here; studies often highlight L-ascorbic acid for its effectiveness, but cost and production needs pull DL-ascorbic acid into the mix.
What the Science Says
Only the L-ascorbic acid part offers real nutritional value. This matters in health conversations, since the body recognizes the L-form and turns it into needed molecules for immune response, wound healing, and keeping tissues fresh. DL-ascorbic acid, by comparison, gets filtered out by the body, so it won’t contribute the same way. Medical experts remind consumers to pay attention to supplement details, especially if they want measurable health benefits.
Looking Ahead: Safer, Smarter Uses
Concerns about purity and effectiveness highlight the need for transparency. Regulatory agencies like the FDA have safety checks in place when DL-ascorbic acid enters food or supplement supply chains. Factories follow strict standards to avoid contamination and make sure dosages stay safe.
It’s tough keeping track of what goes into everything we eat and use, but knowing about ingredients like DL-ascorbic acid puts more power in the hands of shoppers. As more people choose products for both health and safety, reading labels and understanding sourcing turns into a must. Producers willing to invest in research and traceability build trust and help everyone make better choices about what ends up in their cart—or on their skin.
Is DL-Ascorbic Acid the same as Vitamin C?
Vitamin Confusion at the Store
Picking out a vitamin supplement at the pharmacy can get confusing fast. The shelves are packed with ascorbic acids, L-ascorbic acids, and something called DL-ascorbic acid. Most people just want plain Vitamin C for immune support. But the label details matter.
The Science Behind the Name
Vitamin C acts as a crucial nutrient for the body, supporting immune health, helping wounds heal, and guarding cells against oxidative stress. L-ascorbic acid serves as the natural, biologically active form of Vitamin C found in fruits and vegetables—think oranges, kiwis, and bell peppers. This is the form studied for decades, trusted for its absorption and effectiveness in the body.
DL-ascorbic acid, on the other hand, includes both the “L” and “D” forms of ascorbic acid. Chemically, these letters describe the orientation of the molecules. Only the L form provides the well-known health benefits. The D form lacks those benefits and doesn’t function in the body like Vitamin C at all. The combination essentially dilutes the quality: only half is useful, if that. Giving the body a mixed form doesn’t solve any nutritional problems; it just muddies the water.
Labeling and Supplement Choices
Standing in the store, it can be tempting to grab whatever says “ascorbic acid” or boasts a high count of milligrams. Supplement companies sometimes try to stay vague on labels, so consumers assume all ascorbic acid forms give the same results. But the health boost comes from the L-ascorbic acid you get in whole foods and most reputable supplements.
I learned this by looking for affordable options for family members. Some bargain bottles turned out to use DL-ascorbic acid, which costs less to produce. Once I dug into the chemistry, I noticed that only the L form matches the nutrient found in nature—the one research supports. The D form just takes up space in the capsule.
Vitamin C and Real Health Benefits
Countless clinical trials use L-ascorbic acid to study immune support, skin repair, and antioxidant effects in the body. This form passes through cell membranes and acts as an antioxidant, fighting free radicals and supporting enzymatic processes. People who rely on DL forms for the same health benefits aren’t getting what they pay for.
Trust in food-based Vitamin C sources or supplements explicitly labeled as “L-ascorbic acid.” Citrus, berries, and peppers never contain DL-ascorbic acid—nature picks sides. For supplement users who care about boosting health, not all vitamin labels mean the same thing. The difference in one letter can shift real-world results.
Looking for Solutions and Staying Informed
People deserve to know what goes in their bodies. Clear labeling helps shoppers make better choices. More education from pharmacists and health professionals would cut down on confusion. It helps to share these labeling tips with friends and family so they don’t end up with a supplement that doesn’t do the job.
Choosing the right vitamin involves more than reaching for the cheapest bottle. A buyer armed with good information will look for that “L” on the label every time.
What are the potential side effects of DL-Ascorbic Acid?
Understanding DL-Ascorbic Acid
Plenty of folks reach for vitamin C supplements to boost immunity or fill dietary gaps. DL-Ascorbic acid crops up on labels as a synthetic form, a mix of two isomers: L-ascorbic acid and D-ascorbic acid. Most health claims around vitamin C point back to L-ascorbic acid, the natural type used by our bodies. DL-ascorbic acid stands out because the D-form doesn’t show the same vitamin activity, but often gets manufactured for bulk use in everything from supplements to foods.
Common Side Effects, Based on Science and Everyday Experience
It’s easy to think extra vitamin C equals extra health. But problems often come from taking too much, especially in synthetic forms like DL-ascorbic acid. I remember years ago, chasing a quick fix for a winter cold, loading up on vitamin C without thinking twice. It left me with a churning stomach and frequent dashes to the restroom.
Most common side effects stem from the gut. People talk about nausea, upset stomach, heartburn, and diarrhea. The body can only soak up so much vitamin C at a time—doses above 1000 mg often get flushed out fast, leading to loose stools and cramping. The risk grows with higher daily intake, especially in sensitive folks.
Less Common but Noteworthy Concerns
Kidney stones get a lot of attention in discussions about high-dose ascorbic acid. Research points to the formation of oxalate, a breakdown product of vitamin C, which can collect in the kidneys. For those with a history of stones or kidney problems, tapping out at moderate doses matters. Sometimes, synthetic forms might put more strain on kidneys already working overtime.
Some studies have flagged headaches, fatigue, and even mouth ulcers in rare cases. These reactions don’t hit everyone, but those with allergies or existing conditions often find themselves more sensitive. For people who already manage iron overload disorders, vitamin C may kick absorption of iron into overdrive. I've read accounts of folks living with hemochromatosis needing to watch every milligram they eat, as extra vitamin C can tip the balance and drive up iron levels.
Who Should Tread Carefully?
Pregnant women, those who breastfeed, and children should take particular care with DL-ascorbic acid. Their bodies often react differently to high or synthetic forms. People on blood thinners, like warfarin, also need medical advice before starting new supplements since vitamin C can affect how some medicines work.
Working Toward Practical Solutions
Most problems happen when people exceed what the body actually needs. Nurses and doctors often suggest sticking with recommended daily amounts—90 mg for adult men, 75 mg for adult women, a level usually hit with a good diet. For those who supplement, smaller, split doses can lower the chances of side effects. Sourcing supplements from trusted brands, reading labels for synthetic forms, and checking with health professionals become key steps for anyone unsure about what their body can handle.
Natural food sources—like citrus fruits, bell peppers, and broccoli—often deliver a safer vitamin C punch, along with extra nutrients. That tastes better and feels easier on the stomach for most people. Anyone thinking about high-dose supplements, especially in synthetic forms, benefits from asking a doctor and paying attention to how their own body reacts.
How should DL-Ascorbic Acid be stored?
Why Paying Attention to Storage Pays Off
DL-Ascorbic Acid, sometimes called a synthetic version of vitamin C, loses its punch pretty fast if left sitting in the wrong spot. Many people think tossing a container on a pantry shelf works for anything, but that’s not the story with this stuff. Heat, moisture, light—any of those can break it down well before you ever get to use it. Every gram that degrades means less benefit for whatever use you’ve got, whether that’s in supplements, food, or even labs. If the goal is potency and shelf life, where and how you store this powder makes all the difference.
Keep It Cool and Dry
More than once, I’ve seen bulk DL-Ascorbic Acid in storerooms, scooped out with a slightly damp spoon or left open near a sunny window. Facilities like that almost guarantee clumping and discoloration. Water in the air soaks through the tiniest gap in a container, accelerating spoilage. Even in my own kitchen, I’ve seen vitamin C tablets crumble faster in summer if I don’t stow them away from humidity. It’s best to keep this acid tightly sealed in a moisture-resistant container, placed in a refrigerator or, for larger amounts, a cool storage room that stays below 20°C (68°F).
Light Turns Good Powder Bad
One slip many folks make: leaving containers in clear jars or plastic bags near the window. Exposure to sunlight or harsh indoor lighting speeds up chemical changes. These changes aren’t obvious right away—the powder doesn’t always turn a wild color. But lab tests show the loss of ascorbic acid content adds up. Once, working in a food lab, I ran comparison checks for a nutrition facts label and found product stored under regular room lights lost measurable strength in under a month.
Airtight Means What It Says
It’s not overkill to use vacuum-sealed bags or thick, opaque jars with gasket lids. Each time a cap comes off, oxygen gets in, and the game changes. Air starts a slow breakdown process. I’ve used materials like amber glass or high-density plastic—anything that blocks out both air and light—because regular containers let too much through. Consumer safety recalls have called out companies for not meeting label claims just because of careless storage. Trustworthy manufacturers use these kinds of containers for a reason.
Short-Term Versus Long-Term Storage
For home use, a small amber jar tucked away at the back of a cupboard with a moisture absorber works for a few months. For businesses, I’ve seen some just use plastic bags, but any humidity in the air takes a toll. Professional suppliers pack DL-Ascorbic Acid in foil-lined drums with double-seals, then keep them in temperature-controlled warehouses. These details matter. The U.S. Pharmacopeia and the Food Chemicals Codex publish storage guidance for a reason: ignoring it means more waste, expense, and risk to health.
Good Habits Pay Off
Simple habits, like measuring with dry utensils and closing containers fast, preserve quality. In my own work, I’ve seen a difference in shelf life by just adding a silica packet to a jar. This isn’t just for chemists or huge facilities. Every kitchen, café, or supplement maker can put in these prevention steps. They save money, ensure safety, and respect the work that goes into producing pure DL-Ascorbic Acid in the first place.
Is DL-Ascorbic Acid safe for daily consumption?
Understanding DL-Ascorbic Acid
DL-Ascorbic acid sits among those ingredients on food labels that grab your attention, mostly because the name alone sounds industrial. The DL prefix means it’s a mix of two forms—D- and L-ascorbic acid—where L-ascorbic acid matches the natural vitamin C found in fruits and vegetables. Most supplements carry only L-ascorbic acid because it's the one our bodies use. DL-ascorbic acid, in contrast, combines both the active L and the less useful D.
What the Science Really Says
Most conversations about vitamin C refer to the pure L form. In terms of biological activity, research finds that only the L form shows vitamin C effects—boosting the immune system, acting as an antioxidant, and supporting healthy skin. The D form doesn’t get used by the human body in the same way, so when you consume DL-ascorbic acid, you actually get a diluted hit of the vitamin your body expects. Clinical studies confirm it: D-ascorbic acid brings little value. It doesn’t hurt you in reasonable doses, but it doesn't support health the way the L-form does.
Experience matters. Over the years, I have watched supplement trends come and go, and vitamin C has stood the test of time. Health agencies like the FDA and EFSA continue to endorse its safety profile—at the right form and dose. They don’t recommend the synthetic mix, because it’s less efficient and gives no advantage. In fact, taking DL-ascorbic acid daily for the vitamin C boost, you’d need to double up to match the benefits offered by pure L-ascorbic acid.
Safety Risks and Real-World Use
Most manufacturers choose L-ascorbic acid for a reason. Beyond effectiveness, the DL version raises questions about safety in the long run. No major food safety body includes DL-ascorbic acid as a preferred vitamin supplement. Although acute toxicity isn’t reported at low levels, long-term studies are lacking. Consuming the synthetic compound every day, especially if your liver or kidneys already work overtime, might nudge your body out of its comfort zone. Excessive use can contribute to kidney stone formation or digestive distress, mirroring issues seen with mega-doses of L-ascorbic acid.
On a practical level, my approach leans toward established evidence. Real vitamin C—L-ascorbic acid—offers strong proof of safety and value. Citrus fruits, bell peppers, and berries supply plenty of vitamin C in a package our bodies recognize and use efficiently. There’s no reason to complicate matters with a synthetic mix unless there’s a manufacturing need, and that's almost never about consumer health.
Practical Guidance for Consumers
If you’re considering a supplement, scan the label for “L-ascorbic acid.” Brands that use DL-ascorbic acid aren’t common, but the distinction matters. Choose food sources or supplements designed around bioactive forms. For people with special medical circumstances, it always makes sense to check with a healthcare provider before starting any daily supplement.
Nutrition shouldn’t come down to a chemistry experiment. Favor real food. Supplements should fill nutritional gaps, not serve as experiments with unproven compounds. Reliable sources, clear labeling, and sticking to the forms our bodies evolved to use—these are key in making safe and informed choices.
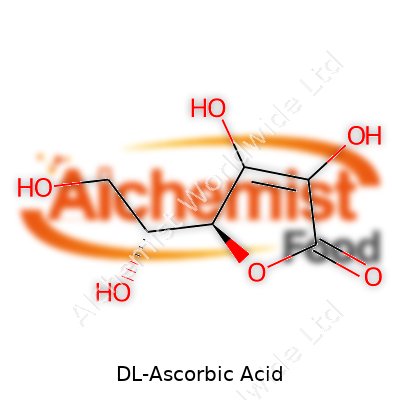
| Names | |
| Preferred IUPAC name | (5R)-5-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one |
| Other names |
DL-Erythorbic Acid Erythorbic Acid Isoascorbic Acid |
| Pronunciation | /diːˈɛl əˈskɔːrbɪk ˈæsɪd/ |
| Preferred IUPAC name | (5R)-5-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one |
| Other names |
Vitamin C Ascorbate 3-Keto-L-gulofuranolactone L-threo-Hex-2-enono-1,4-lactone |
| Pronunciation | /diːˈɛl əˈskɔːrbɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 50-81-7 |
| Beilstein Reference | 4032457 |
| ChEBI | CHEBI:38290 |
| ChEMBL | CHEMBL50 |
| ChemSpider | 546 |
| DrugBank | DB00126 |
| ECHA InfoCard | 05614b0c-cd1c-4f3a-af69-bf6b8f4b7d5c |
| EC Number | EC 200-066-2 |
| Gmelin Reference | 82537 |
| KEGG | C00072 |
| MeSH | D002207 |
| PubChem CID | 54670067 |
| RTECS number | CI7655000 |
| UNII | X50L779A7F |
| UN number | UN3077 |
| CAS Number | 50-81-7 |
| Beilstein Reference | 1724225 |
| ChEBI | CHEBI:38290 |
| ChEMBL | CHEMBL50 |
| ChemSpider | 5684 |
| DrugBank | DB00126 |
| ECHA InfoCard | 03e4ffee-88f2-49e7-95f0-0e228c64c572 |
| EC Number | EC 200-066-2 |
| Gmelin Reference | 1015 |
| KEGG | C00072 |
| MeSH | D002388 |
| PubChem CID | 54670067 |
| RTECS number | WS7250000 |
| UNII | WQX9C7F6PO |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C6H8O6 |
| Molar mass | 176.12 g/mol |
| Appearance | white or almost white crystalline powder |
| Odor | Odorless |
| Density | 1.65 g/cm³ |
| Solubility in water | freely soluble |
| log P | -1.85 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.2 |
| Basicity (pKb) | 8.34 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.62 |
| Dipole moment | 2.51 D |
| Chemical formula | C6H8O6 |
| Molar mass | 176.12 g/mol |
| Appearance | White crystals or crystalline powder |
| Odor | odorless |
| Density | 1.65 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.85 |
| Acidity (pKa) | 4.10 |
| Basicity (pKb) | 8.34 |
| Magnetic susceptibility (χ) | -6.2×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.65 |
| Dipole moment | 2.5646 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 181.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1225.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1100 kJ/mol |
| Std molar entropy (S⦵298) | 146.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1094.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1921 kJ/mol |
| Pharmacology | |
| ATC code | A11GA01 |
| ATC code | A11GA01 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes mild skin irritation. |
| GHS labelling | GHS07, Warning, H315, H319, H335 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid breathing dust. Wash hands thoroughly after handling. Use personal protective equipment as required. |
| Flash point | 141°C |
| Autoignition temperature | 660°C |
| Lethal dose or concentration | LD50 Oral (Rat): 11900 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 11,900 mg/kg |
| NIOSH | AS2475000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | Not listed |
| Main hazards | May cause respiratory and digestive tract irritation. May cause eye and skin irritation. |
| GHS labelling | GHS07, Exclamation mark |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Use personal protective equipment as required. Wash thoroughly after handling. Do not eat, drink, or smoke when using this product. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 129 °C |
| Autoignition temperature | 660°C |
| Lethal dose or concentration | LD50 oral rat 11900 mg/kg |
| LD50 (median dose) | 11900 mg/kg (rat, oral) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of DL-Ascorbic Acid: Not established |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | No IDLH established |
| Related compounds | |
| Related compounds |
L-Ascorbic acid Sodium ascorbate Calcium ascorbate Magnesium ascorbate Ascorbyl palmitate Dehydroascorbic acid |
| Related compounds |
Ascorbic acid phosphate Ascorbyl palmitate Erythorbic acid Dehydroascorbic acid Calcium ascorbate Sodium ascorbate |

