Disodium Pyrophosphate: A Commentary Rooted in Fact and Experience
Historical Development
Disodium pyrophosphate caught attention in the twentieth century when the food industry needed solutions for leavening and preservation. Industrial chemistry’s progression after World War II led manufacturers to refine phosphate production, turning early crude mixtures into the more consistent, pure product seen today. Earlier generations faced unpredictable behavior in baking and processing, sparking steady demand for functional additives. Over time, as regulatory bodies tightened food safety requirements and public awareness of additives deepened, a push for greater transparency and finely tuned specifications emerged. Companies in Asia, Europe, and North America built dedicated plants as global demand for convenience foods boomed, cementing disodium pyrophosphate’s role in modern food technology alongside its growth in other sectors.
Product Overview
Disodium pyrophosphate, or SAPP by industry shorthand, stands as a reliable leavening agent and sequestrant found across industries. Its primary role is to interact with sodium bicarbonate in dough or batter, forging the bubbles that lift pancakes or bread. Commercial grades vary based on their reactivity and purity, tailored for diverse uses in food, ceramics, and cleaning products. Despite many names—sodium acid pyrophosphate being another—end users recognize it for one thing: performance. From breakfast tables to factory floors, the demand revolves around consistency and predictability. Packaged materials shipped to industrial customers must display batch numbers, grade codes, and handling instructions, aligning with legislation in the United States, European Union, and Asia-Pacific regions.
Physical & Chemical Properties
This white, crystalline powder resists flowing freely unless dried, drawing water from air thanks to its hygroscopic nature. It dissolves easily in water, reaching full potency fast, which matters for bakers and food processors balancing precise timing and consistency. The chemical formula Na2H2P2O7 and a molecular weight near 221.94 g/mol give it a profile suited for processing plants and lab work. SAPP’s ability to interact with metal ions improves cooking results, such as preventing gray discoloration in potatoes or stabilizing canned seafood. Not all grades have identical solubility or grain size—plants tweak these for shelf life or blendability, which shapes how it enters recipes or manufacturing processes.
Technical Specifications & Labeling
Producers and regulatory agencies expect tight control over technical specifications for disodium pyrophosphate. Impurity levels (arsenic, heavy metals, fluorides) stay well below thresholds set by Codex Alimentarius and local food agencies. Besides standard purity benchmarks above 95%, labels must note grade (food, technical, analytical), origin, date, and lot number. For food use, items carry identifiers like E450a (Europe) or INS 450 (international). End buyers scrutinize not just content but labeling, since compliance affects import permits and bulk transactions. Factories performing third-party audits put extra attention on shipment certifications and the traceability chain, protecting both downstream users and the brand reputation.
Preparation Method
Manufacturers synthesize disodium pyrophosphate by heating monosodium phosphate to about 200–350°C. This process triggers dehydration, joining two phosphate molecules with a pyrophosphate bond. Until modern automation took over, batch-to-batch consistency challenged producers. Now, precise temperature controls and continuous processing reactors push the reaction efficiency higher, squeeze down waste, and cut resource use. Some plants opt for innovative catalysts or recycling of by-products, minimizing their footprint and supporting circular chemistry. Plant managers monitor effluents and energy use, recognizing the ongoing environmental scrutiny facing phosphate chemistry. Storage conditions—cool, dry, away from acids—matter from factory to distribution.
Chemical Reactions & Modifications
In the lab and on the production floor, disodium pyrophosphate reacts with alkaline substances such as sodium bicarbonate, releasing carbon dioxide—key for bakery rise. Its sequestrant properties shine when it binds calcium, magnesium, or iron, stopping unwanted browning or textural changes in food. Developers sometimes generate multipart leavening systems by pairing SAPP with other phosphates, fine-tuning the reaction rate and timing in dough. For non-food applications, chemical plants combine SAPP with surfactants to make rust removers, cleansers, or detergents more effective. Modified pyrophosphates occasionally appear in specialty ceramics or flame retardants, broadening the application landscape based on collaborative industry research.
Synonyms & Product Names
Products often appear under names such as SAPP, sodium acid pyrophosphate, or food additive E450a. Suppliers across the globe market with local variations, but most stakeholders recognize the core chemical. Trade names shift with branding decisions, yet technical sheets routinely highlight disodium pyrophosphate for clarity—ensuring cross-market communication and purchasing aligns expectations. Some large producers run their own brands, granting premium guarantees or traceability to source, adding confidence for international procurement teams seeking reliability and supply security.
Safety & Operational Standards
Safety management ranks high along the production chain. Exposure guidelines from OSHA, NIOSH, and comparable organizations in Europe dictate air monitoring in packaging and handling zones. Workers involved in transfer or mixing use dust masks, eye shields, and gloves, as fine powder exposure can irritate mucous membranes and skin. Rigorous training for spills or accidental release ensures quick mitigation, minimizing harm to people or site operations. Facilities include ventilation and dust-collection equipment, reviewing protocols regularly to anticipate regulatory updates on phosphates. Food contact surfaces require scheduled washing, guarding end users from cross-contamination. Many customers demand Globally Harmonized System (GHS) labeling and audit reports to prove commitment beyond minimum rules.
Application Area
Bakers and snack manufacturers rely on SAPP to control leavening speed for cakes, pancakes, biscuits, and even dumplings—timing stays predictable, reducing waste and off-batches. Potato processors use SAPP to hold natural color, keeping french fries golden rather than brown. Seafood packers find value as SAPP binds minerals, preserving texture and minimizing spoilage in canned fish and shellfish. Outside the grocery aisle, ceramicists improve formation and firing by adding SAPP to mineral slurries, affecting grain packing and final product density. Metalworkers tap into rust removal properties, leveraging phosphate’s chelation to clean and prep surfaces ahead of painting or coating. Further, SAPP enters formulas for household and industrial detergents, expanding cleaning effectiveness by softening water and boosting surfactant performance.
Research & Development
Academic and industrial teams both drive ongoing study of disodium pyrophosphate. Researchers dive into food texture, shelf life, and nutrient release, keeping pace with evolving diets and regulatory changes. Material scientists map out new uses in ceramics or flame retardants, blending SAPP with other additives for unique performance features. Environmental scientists pay close attention to phosphate runoff, spurring innovation in closed-loop recycling and alternative sourcing. As consumer health trends shift toward cleaner labels, R&D groups experiment with blending SAPP at lower usage rates or pairing with natural stabilizers, banking on improved public trust and market access. At conferences, results get exchanged across continents, showing how nimble adaptations to SAPP technology ripple through industry supply chains.
Toxicity Research
Toxicological studies in animals and simulated environments provide data for regulatory limits and usage levels. Most food safety agencies classify SAPP as safe at specified dosages, focusing on acute or chronic exposure risk. Long-term intake above limits can disturb mineral metabolism, so rules set acceptable daily intake based on human and animal data. Toxicologists monitor phosphate levels in diets and release periodic reviews, supporting product label updates and public awareness. Research recognizes that while rare, hypersensitivity can occur, driving industry vigilance on cross-contamination and warning labels for sensitive consumers. Environmental reviews also address bioaccumulation and watercourse contamination, placing pressure on manufacturers to document disposal and ensure responsible supply.
Future Prospects
Looking forward, demand for disodium pyrophosphate will reflect shifts in processed foods, global diets, and regulatory scrutiny. As plant-based and “cleaner label” food trends gather force, companies explore reducing total phosphate use, experimenting with functionally equivalent blends that keep quality steady. In parallel, the rise in ceramic, industrial cleaning, and water treatment needs outside food points to new growth areas. Technology holds promise for energy-efficient synthesis routes, slashing production costs and carbon footprints. Digital tracking will likely improve supply transparency, reassuring customers on origin and safety. Lastly, more sustainable phosphate sourcing—including by-product repurposing or mineral recycling—lines up with environmental accountability, shaping the next generation of SAPP producers and users worldwide.
What is Disodium Pyrophosphate used for?
Life With Additives: Where Disodium Pyrophosphate Comes Into Play
Every time I browse the ingredients list on a bag of frozen hash browns or a can of baked beans, I expect to see more than just potatoes or beans. Disodium pyrophosphate, or DSPP, pops up pretty often. It doesn’t sound appetizing, but it has a real impact on how that food looks and holds together by the time it hits your plate. Most people haven’t thought twice about it, but I’ve followed its trail out of curiosity and a bit of concern over additives in my own family’s meals.
Food Quality and Safety: More Than Cosmetic
Potatoes may turn gray after peeling and cutting because of oxidation. DSPP acts fast, locking in that pale, fresh color before gray patches settle in. This helps keep big food brands delivering a tidy, appetizing product. The same principle gets used in seafood products, pancake mixes, and baking powders. Without DSPP, those fluffy pancakes from the corner diner might turn out flat and pale, and canned tuna might lose its delicate appeal.
Science backs up these uses. Studies show DSPP alters the acidity and minerals in foods, preventing the chemical reactions behind ugly browning and texture loss. Still, the main benefit lies in how it lets factories process food at large scale while still producing something people want to eat.
Baking Powder and Leavening
Bakers often use DSPP because of what it does in recipes that rely on chemical leavening. As a leavening acid, it helps release carbon dioxide from baking soda. That gas puffs up muffins and biscuits, leading to that airy crumb my kids hope for in every bite of store-bought snacks. Without DSPP, baked goods can fall flat—not just in the oven, but in sales, too.
Health Questions and Regulatory Oversight
Thinking about food additives leads to healthy skepticism. I don’t want mystery chemicals sneaking into my meals—my parents were the same way. Fortunately, agencies like the FDA and European Food Safety Authority review ingredients like DSPP regularly. Every time a question comes up, such as phosphate levels contributing to kidney problems or cardiovascular risks, these organizations run new safety reviews. So far, they’ve set clear use limits, and the research on DSPP keeps it well below levels considered harmful, even for people eating a standard processed food diet.
Do We Need It? Exploring Alternatives
Some folks chase fresher, less processed foods because they want fewer additives overall. That’s the direction I try to steer my own shopping cart most weeks. Yet, not everyone has time or money to peel and cook from scratch, so food companies keep using DSPP for its reliability. Some smaller producers have tried ascorbic acid or citric acid as substitutes for keeping food color, but these rarely bring the same effect in high-volume production lines or complex products.
Moving Toward Transparency in Food Choices
Reading about DSPP shaped my own approach to food as much as any diet trend or health scare. Discussion seems more useful than panic. Real change probably means customers keep reading ingredient lists, companies keep improving, and regulators keep an eye on science as it develops. Shoppers deserve clear labels, straightforward science, and some trust built by regular safety checks. Deciding what to keep on our plates grows easier and smarter with that kind of openness and engagement.
Is Disodium Pyrophosphate safe to eat?
Getting Real About What’s in Your Food
People want to know what all those hard-to-pronounce ingredients really mean for their health. Disodium pyrophosphate pops up in a lot of packaged foods, from frozen hash browns to canned fish. Some worry when they see chemicals on ingredient labels—worries that usually come down to safety and possible health risks. Questions like that feel important, since nearly everyone grabs something quick and processed at some point.
What is Disodium Pyrophosphate?
This powdery white stuff helps keep food looking and tasting good. Companies use it to prevent potatoes from turning gray, leaven baked goods, and keep meats tender. It’s also in some baking powders. Most people have eaten much more of it than they realize. It’s made up of sodium and phosphorous, both of which our bodies need. The double “pyro” part just means two phosphate groups are stuck together.
What Scientists and Regulators Say
The US Food and Drug Administration (FDA) calls disodium pyrophosphate “generally recognized as safe” (GRAS). That means experts agree this stuff shouldn’t pose health risks under normal use, the way it’s added to foods now. The European Food Safety Authority has also reviewed the science and found the amount people usually get in food is well below any level that might cause harm. A 2019 assessment set a safe intake limit of 40 milligrams per kilogram of body weight per day for phosphate additives combined, including disodium pyrophosphate. Most folks don’t come close to that number in their daily meals.
Concerns About Phosphates
There’s some anxiety about eating too many phosphates. People with healthy kidneys quickly get rid of the extra, but someone with kidney disease can struggle to clear out that much phosphorus. High blood levels might cause problems with bones and heart over time. If you’ve never had kidney problems, that’s rarely an issue. Still, nutritionists sometimes nudge people to cook fresh foods more often, which naturally means eating less of all kinds of additives, including phosphate ones. It’s less about any single chemical and more about keeping your diet balanced and not relying on ultra-processed foods to run your week.
How to Make Informed Choices
Reading ingredient lists and nutrition labels always helps. If you notice a lot of additives (not just disodium pyrophosphate), it’s worth taking a step back and thinking about why you’re eating that product. No evidence points to small amounts of disodium pyrophosphate causing harm to most people, but nobody loses out by cooking their own meals more often. Many food chemicals have names that sound scarier than what they actually do, and government agencies perform constant reviews to make sure new evidence doesn’t slip through the cracks.
Thinking Big Picture
Every year, researchers and regulators keep a close watch on food safety. To stay in the safe zone, most people can focus on variety and moderation in what they eat. Looking back at my own habits, grabbing a bag of frozen potatoes a few times a month saves time, but my shopping cart always gets some produce and grains, too. There’s room for convenience sometimes—as long as it’s not running the whole show. That kind of eating pattern tends to keep you well within limits for food additives like disodium pyrophosphate, with no need to stress unnecessarily.
Is Disodium Pyrophosphate vegan or vegetarian?
The Place of Disodium Pyrophosphate in Plant-Based Eating
Every trip to the grocery store, shoppers bump into ingredients with long names. One of these is disodium pyrophosphate, shortened as DSPP on food labels. It plays a role in baking powders, vegan meats, and even those frozen fries you pull out of the bag when you’re tired. For anyone looking to avoid animal products, one question crops up: should disodium pyrophosphate make the green-light list?
How Companies Make Disodium Pyrophosphate
DSPP comes from reacting food-grade sodium carbonate with phosphoric acid, a substance that often gets sourced from phosphate rocks deep underground. The result? A salt with no connection to animals or dairy. From a chemical point of view, nothing in it comes from a living creature, fish, insect, or mammal. Manufacturers follow strict quality standards for food chemicals set by food safety authorities in places like the United States and the European Union. The process stays within the world of minerals and acids—no animal bones or eggshells end up in the final mix.
Is DSPP Vegan and Vegetarian?
Plenty of vegans think about trace ingredients in processed food and personal care products. For them, tracing each additive can feel like detective work with a magnifying glass. Disodium pyrophosphate, based on most publicly available documentation and food agency listings, fits both vegan and vegetarian guidelines. It isn’t an animal byproduct, and no animal suffered in its production.
I’ve talked to plant-based eaters and dietitians who comb through ingredient lists regularly. Cruise through online vegan forums, and the verdict often repeats itself: DSPP doesn’t trigger alarms for animal-derived risks the way gelatin or casein does. These food experts treat it as a simple mineral salt, found in dozens of everyday groceries from crackers to canned beans. Vegan certification programs and food-safe organizations usually nod it through their requirements for plant-only foods.
What’s the Catch?
Some people ask about other issues—environmental impact or whether a product got tested on animals. Right now, no credible reports connect DSPP to animal testing for food purposes. Environmental impact ranks as a concern for all phosphate-based additives, since mining for phosphates can harm waterways and habitats. That sparks a larger ethical conversation, one tied to global agriculture and big business. Phosphates support crop growth and end up in lots of basic fertilizers, so the story doesn’t begin or end with food additives.
Choosing for Your Values
Those who stick to a vegan or vegetarian lifestyle often chase more than chemistry—they care about supply chain transparency, worker safety, and planetary health too. Looking at DSPP, most authorities and community voices call it vegan and vegetarian. If trace processing steps trouble you, reaching out to a favorite brand or digging into its manufacturing sources might help. Some companies respond to questions about additives, especially when customers raise concerns about animal products or ethical sourcing. Labels sometimes leave you guessing, but collective consumer pressure often shifts companies toward openness.
Simple Solutions for Shoppers
If uncertainty lingers about any processed ingredient, sticking with whole foods—fruits, grains, nuts, and fresh veggies—sidesteps food science jargon entirely. For everyone else, DSPP lands safely in the vegan and vegetarian camp based on what food science knows today. Being informed and keeping conversation lines open with brands pushes the food world closer to honesty and transparency for all eaters.
What foods commonly contain Disodium Pyrophosphate?
What Disodium Pyrophosphate Does
Disodium pyrophosphate shows up in more foods than people might guess. This ingredient helps with texture, prevents certain color changes, and leavens baked goods. Food scientists reach for it because it works, not because anyone is gunning for complex chemistry for fun. As a food writer with a history of peering at packaging, I keep bumping into this additive in both snack aisles and the frozen section. Some cooks might not even notice it, but it's there, quietly shaping foods in the background.
Baked Goods and Pancake Mixes
For anyone who loves fluffy pancakes or biscuits out of a box, chances are that disodium pyrophosphate played a part. It fires up the leavening process, giving rapid lift to quick breads. Without it, that “just right” texture wouldn’t show up so easily. Food chemistry often gets blamed for “weird stuff,” but as someone who’s tried to get tall biscuits without baking powder—good luck.
Processed Potatoes: French Fries, Hash Browns, and Mashed Potato Flakes
Another spot this compound sneaks in: potato products. Whether it’s fries in fast food, frozen hash browns, or potato flakes for instant mash, disodium pyrophosphate stops the gray tint that potatoes like to show after processing. Most people probably don’t crave gray mash, so this step matters for both taste and trust. You’ll see it in ingredient lists to keep spuds looking like, well, spuds.
Canned Seafood and Poultry
Canned tuna, crab, or chicken—these are frequent users. Disodium pyrophosphate keeps these proteins from turning an odd color. From my own experience growing up with canned tuna lunches, the appearance sells the meal. If things look off, the can stays closed. Fact is, color attracts lot of eaters before taste ever gets a chance.
Processed Meats and Sausages
Look at labels for deli meats, hot dogs, or sausages. You’ll almost always find disodium pyrophosphate there. Here it lends not only to color retention but helps hold moisture. I’ve worked behind the counter slicing ham—no one wants a dry, dull ham sandwich. It helps that the slices stay pink too.
Frozen or Packaged Doughs
Pie crusts, cookie dough logs, pizza doughs—these freezer and refrigerated favorites often contain a dash of chemical leaveners. Disodium pyrophosphate teams up to give doughs that nice “pop” in the oven. Try baking pre-made crusts and skipping out on this chemistry trick: odds are the difference shows.
Breakfast Cereals
Some cereals, especially ones promising crunch or colorful marshmallows, lean on disodium pyrophosphate for structure and appearance. I’ve noticed that those rainbow-hued cereals and some oat-based shapes perform better in milk because of tweaks like these.
Questions About Safety and Solutions
Some shoppers worry about long-named ingredients. Disodium pyrophosphate has earned approval as safe in regulated amounts by the FDA and equivalent groups in Europe. The real debate circles around processed food and how much anyone should rely on engineered meals. Swapping boxed for home-cooked recipes using basic ingredients limits these additives. For those reading labels, sticking with foods that have short, familiar ingredient lists can help avoid excess. More transparent labeling and education make a difference—to both choice and confidence at the table.
Does Disodium Pyrophosphate have any side effects?
Understanding What You’re Eating
Standing in a grocery store, reading the fine print on a bag of frozen hash browns or canned seafood, you often see the tongue-twister “disodium pyrophosphate.” Food makers use it to help baked goods rise, keep potatoes from turning gray, and keep preserved meats looking bright instead of dull. On paper, this chemical works wonders for getting food to look and taste just like you expect. But hearing the word “phosphate” raises an eyebrow. Is there a price for all that convenience?
What Science Tells Us About Safety
Disodium pyrophosphate gets the green light from the U.S. Food and Drug Administration and the European Food Safety Authority for safe use in food products. Most people chew through tiny amounts in processed foods, and these levels don’t raise flags in short-term studies. The World Health Organization sets its daily acceptable intake at 70 mg phosphate per kilogram of body weight. That’s more than most people would get from an ordinary diet, even if they eat a fair share of frozen waffles or instant mashed potatoes.
Potential Side Effects
Too much phosphate doesn’t just leave your system quietly. Your kidneys have to work harder to flush out any extra. Given enough phosphate, your body might see shifts in calcium balance, which could impact bone strength over decades. Day to day, sensitive folks—especially those with chronic kidney disease—can run into higher risks. High phosphate can build up, which throws off the balance of minerals and may increase chances of heart and bone problems.
Some people have shared stories about stomach upset after eating foods rich in food additives, including disodium pyrophosphate. The research in healthy adults doesn’t show big reasons to worry about occasional exposure, but for those managing kidney issues, the advice is different: limiting phosphate intake matters more.
Real-World Impacts
Growing up in a family with diabetes and kidney troubles, you get used to looking at food labels for carbohydrates and sodium. Now, phosphate is another name to watch. Conversations with doctors and dietitians point out that people with impaired kidneys can’t clear out phosphates as quickly. Even without obvious symptoms, long-term damage might sneak up if you’re not careful.
Looking at trends, one-third of food in the American grocery store now contains added phosphates. Most people don’t see trouble from that, but this change makes it easy to eat more than nature intended, especially if you reach for lots of processed snacks. Chronic high phosphate intake, partners with sugary drinks and sodium, stresses the body in new ways—a trend public health experts watch closely.
What Can You Do?
Turning the package over to check for disodium pyrophosphate and other additives is a small step with big potential benefits, especially for people at higher risk of mineral imbalances. Choosing more whole, unprocessed foods creates fewer landmines for those with kidney concerns or who want to play it safe.
Simple habits, like rinsing canned goods or picking fresh or frozen vegetables, add up. Dietitians recommend counting phosphate alongside sodium and sugar for people who have kidney disease or a family history of bone issues. Food scientists and the public health team continue to push for clearer labels so shoppers can decide what belongs on their plate.
Overall, disodium pyrophosphate helps keep food looking appetizing and lasting longer, but it’s not invisible to your body. Knowing its role encourages us to keep balance in our diets and respect our kidneys’ hard work.
| Names | |
| Preferred IUPAC name | Disodium dihydroxy-dioxido-oxo-diphosphate |
| Other names |
Sodium acid pyrophosphate Disodium dihydrogen pyrophosphate SAPP Disodium diphosphate Disodium phosphate (pyrophosphate) E450(i) |
| Pronunciation | /daɪˈsoʊdiəm paɪrəˈfɒsfeɪt/ |
| Preferred IUPAC name | disodium diphosphate |
| Other names |
Sodium acid pyrophosphate SAPP Disodium dihydrogen diphosphate Disodium diphosphate Disodium phosphate (pyrophosphate) |
| Pronunciation | /daɪˈsoʊdiəm paɪˈrɒfəˌsfeɪt/ |
| Identifiers | |
| CAS Number | 7758-16-9 |
| Beilstein Reference | 3977559 |
| ChEBI | CHEBI:45044 |
| ChEMBL | CHEMBL1201191 |
| ChemSpider | 14232 |
| DrugBank | DB11080 |
| ECHA InfoCard | ECHA InfoCard: 03-2119488967-14-0000 |
| EC Number | 231-767-1 |
| Gmelin Reference | 8738 |
| KEGG | C18683 |
| MeSH | D005408 |
| PubChem CID | 24814 |
| RTECS number | UW9100000 |
| UNII | 18X9G63N3T |
| UN number | UN 3077 |
| CompTox Dashboard (EPA) | DTXSID3023503 |
| CAS Number | 7758-16-9 |
| Beilstein Reference | 474130 |
| ChEBI | CHEBI:18338 |
| ChEMBL | CHEMBL1201191 |
| ChemSpider | 8456 |
| DrugBank | DB11074 |
| ECHA InfoCard | ECHA InfoCard: 030-040-00-2 |
| EC Number | 231-767-1 |
| Gmelin Reference | 7976 |
| KEGG | C06223 |
| MeSH | D010939 |
| PubChem CID | 24813 |
| RTECS number | UX8130000 |
| UNII | 18W8G9IC1V |
| UN number | UN 3278 |
| Properties | |
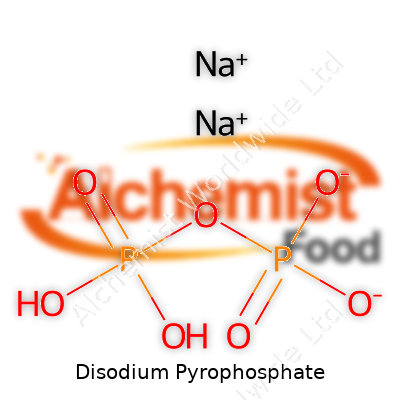
| Chemical formula | Na2H2P2O7 |
| Molar mass | 221.94 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.86 g/cm³ |
| Solubility in water | Soluble |
| log P | -4.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.0-2.0 |
| Basicity (pKb) | 1.0 |
| Magnetic susceptibility (χ) | −81.0×10⁻⁶ cm³/mol |
| Dipole moment | 4.64 D |
| Chemical formula | Na2H2P2O7 |
| Molar mass | 221.94 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.86 g/cm³ |
| Solubility in water | Soluble |
| log P | -4.71 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 7.2 |
| Magnetic susceptibility (χ) | -54.0e-6 cm³/mol |
| Refractive index (nD) | 1.46 |
| Viscosity | water-thin |
| Dipole moment | 2.18 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 229.0 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -2428.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2877.8 kJ/mol |
| Std molar entropy (S⦵298) | 206.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2428.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −2887.6 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | E450 |
| ATC code | E450 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Wash hands thoroughly after handling. |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 Oral Rat 2650 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,980 mg/kg (rat, oral) |
| NIOSH | NT8050000 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 0-70 mg/kg (as P₂O₇²⁻) |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (oral, rat): 2650 mg/kg |
| LD50 (median dose) | LD50 (median dose) for Disodium Pyrophosphate: 3980 mg/kg (oral, rat) |
| NIOSH | WF8580000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 0.1 g/kg |
| Related compounds | |
| Related compounds |
Monosodium phosphate Disodium phosphate Trisodium phosphate Tetrasodium pyrophosphate |
| Related compounds |
Monosodium phosphate Disodium phosphate Trisodium phosphate Tetrasodium pyrophosphate Sodium triphosphate Sodium hexametaphosphate |

