Disodium Dihydrogen Pyrophosphate: Past, Present, and Future
Historical Development
Disodium dihydrogen pyrophosphate, known in labs and factories as SAPP, has a story stretching back to the early days of inorganic phosphate chemistry. Chemists in the 19th century tinkered with combinations of phosphoric acids and alkaline metals, hunting for compounds that offered more than simple stabilization. By the 1950s, the food and baking industries noticed that SAPP could transform leavening reactions, doing something that simple baking soda or straight phosphates couldn’t replicate. The compound found its way into commercial flour mills and bakeries, especially in the post-war era when food manufacturers wanted longer shelf life and consistent results from ready-mixes. European and North American regulations around food safety further pushed research into additives that could improve function with low health risk, helping SAPP find global adoption in professionally baked goods, cured meats, and cleaning agents.
Product Overview
This colorless, crystalline compound pops up on food and chemical labels across several industries. Its main chemistry taps into acidity regulation and controlled release of gases. In plain English, it helps dough rise evenly and slows oxidation in foods, which helps keep processed items fresh and appetizing. Beyond food, SAPP acts as a dispersing agent and sequestering agent, which boosts cleaning efficiency in detergents and improves quality in oil drilling and ceramic manufacturing. Both granular and powder forms circulate through factories, with grades tweaked for specific industrial or food uses.
Physical & Chemical Properties
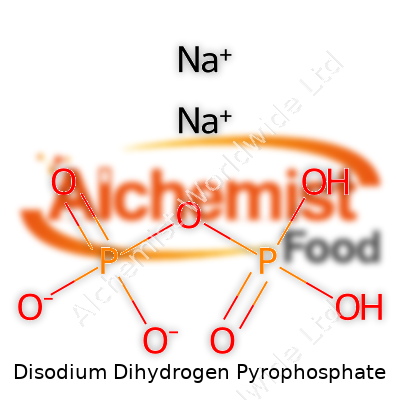
With a molecular formula of Na2H2P2O7 and a molar mass just over 221 g/mol, SAPP appears as white granules or powder. Highly soluble in water, it slips effortlessly into doughs, batters, and cleaning mixtures. SAPP breaks down above 200°C, and this heat dependence makes it fit well into baking cycles. Its slightly acidic pH—typically between 4.0 and 4.8 in solution—counters the alkalinity of baking soda, so together they regulate both flavor and final product texture. The compound resists clumping on the shelf, which matters when long production lines and hot warehouses might cause lesser ingredients to cake up and lose function.
Technical Specifications & Labeling
Food-grade SAPP usually promises purity above 95%, with tight controls on heavy metals and contaminants set by organizations like FAO/WHO and the US FDA. Labels in North America usually list it as Disodium Dihydrogen Pyrophosphate, SAPP, or by its E number: E450(i). Exact labeling requirements depend on use: in baking powders, it often appears alongside sodium bicarbonate; in processed meats, it’s listed near curing agents. Companies keep a close eye on water content and particle size, since deviations can shift leavening rates or affect how SAPP disperses through a mixture. Bulk packaging often holds between 25 kg and one metric ton, in polyethylene-lined drums or reinforced bags to block moisture.
Preparation Method
Industrial synthesis of SAPP usually starts with sodium carbonate and food-grade phosphoric acid. Chemists mix and heat these reagents to push a condensation reaction: two equivalents of phosphoric acid combine, losing water and leaving behind the pyrophosphate group. Careful temperature control keeps the yield high and suppresses unwanted byproducts like tripolyphosphate. The solution gets cooled, then crystallized—either in open evaporators or vacuum chambers, depending on purity needs. After filtration and drying, the crystals are milled and sieved to desired size, then packed for shipping. Safety checks screen for residual acid or carbonate and ensure lot-to-lot consistency.
Chemical Reactions & Modifications
In baking, SAPP teams up with sodium bicarbonate, releasing carbon dioxide at a steady rate as doughs warm and rise. This staged reaction gives time for heat penetration and gluten structuring, as opposed to flash-leavening, which can collapse batters. SAPP may also undergo partial hydrolysis in high-moisture products, where it breaks down to sodium phosphate forms that further buffer acidity. Chemists sometimes blend SAPP with other leavening acids to tune the “rate of reaction” profile for specific products—yielding fast, slow, or dual-acting baking powders.
Synonyms & Product Names
The compound shows up as Disodium Pyrophosphate, Sodium Acid Pyrophosphate, Sodium Diphosphate, SAPP, and E450(i), depending on the regulatory region. Chemical suppliers list variations to flag minor differences in particle size or moisture content. Specialty grades go by names like “Slow-Reacting SAPP” or “High-Solubility SAPP.”
Safety & Operational Standards
Factory workers require gloves and dust masks while handling SAPP to prevent skin and respiratory irritation, even though ingestion at approved levels shows low toxicity. Storage sites keep the material in dry, cool areas since exposure to moisture can cause caking and lessen performance in blends. Global food regulators—Codex Alimentarius, the FDA, the European Food Safety Authority—agree on safe upper limits for SAPP in food, usually in the range of several grams per kilogram, much less than former generations of phosphates. Workers assess dust levels in air at regular intervals to keep exposure below recommended occupational limits. Transport follows Hazard Class protocols for irritant powders, and product labels in multiple languages alert handlers to avoid unnecessary contact.
Application Area
Food processors rely on SAPP in self-rising flours, ready-to-cook pancake and cake mixes, and refrigerated biscuit dough. Anybody who’s ever baked from a box owes the texture—the even crumb, the lift—to SAPP’s fine-tuned gas release. Processed meats like hot dogs and deli ham hold pink color and moisture with a pinch or two of SAPP, slowing nasty color changes and giving a consistent bite. Outside of food, SAPP strengthens ceramic glazes, keeps liquid detergents flowing smoothly (no unsightly chunks forming in the bottle), and boosts oil recovery by managing hard water ions in drilling fluids. Some toothpaste brands use it to control tartar and prevent clogging in manufacturing lines.
Research & Development
Scientists in academia and private industry keep searching for ways to improve SAPP’s function or cut costs in manufacturing. Nutritional researchers probe diets high in phosphate-food additives for links to kidney health and cardiovascular disease, driving some consumers to watch these labels more closely. Researchers in polymer and ceramic fields experiment with SAPP in new binding agents, testing whether it can improve strength while cutting reliance on more expensive or environmentally risky ingredients. Food technologists hunt for natural alternatives that match SAPP’s reliability, but few have found a plant or mineral extract that can hold up against the consistency demanded by industrial-scale bakers and meat processors.
Toxicity Research
Extensive toxicological studies support SAPP’s use as a food additive. Rodent trials show high safety margins, with adverse effects only at doses thousands of times above realistic dietary intake. Long-term human studies reveal no ties to cancer or direct organ harm at typical consumption levels, though nutritional scientists debate wider health effects of excessive phosphate in Western diets. Kidney specialists warn that certain patients with advanced renal disease might better watch out for added phosphate in foods, SAPP included. Regulatory agencies worldwide reset maximum allowable limits and demand fresh safety reevaluations every few decades as new data rolls in.
Future Prospects
With the processed food sector pressing for cleaner labels, future developments may see SAPP modified to lower sodium contribution or to blend with natural acids for hybrid leavening systems. Environmental regulations could reshape manufacturing protocols, phasing out phosphates in certain detergents and fertilizers, which may slow SAPP’s use outside food and ceramics. Increased transparency in labeling and a consumer shift to plant-centered diets will drive further research into functionally equivalent, sustainably sourced alternatives. Companies investing in cleaner raw materials and zero-waste processing tend to find loyal markets, especially in regions where food safety and sustainability guide purchasing habits. Every generation of researchers, from flavor chemists to material scientists, will keep finding new twists on this reliable compound.
What is Disodium Dihydrogen Pyrophosphate used for?
What’s in Your Pantry?
The name Disodium Dihydrogen Pyrophosphate draws attention for its length alone. For many, this ingredient pops up at the end of an ingredient list on boxed foods. It leaves people scratching their heads, wondering how a chemical-sounding name ends up in everyday items like cake mix, potato products, or canned seafood. Food science doesn’t aim to scare anyone; it wants consistency, safety, and a product that looks like it does on the box. This ingredient became a mainstay for a simple reason: it just works in ways real food sometimes falls short.
What Does It Do in Food?
Disodium Dihydrogen Pyrophosphate steps in as a leavening acid in baking powders and instant dough mixes. In my old job at a bakery supply warehouse, this stuff was everywhere. It reacts with baking soda and gives CO₂, helping doughs and batters puff up without waiting on yeast. That means pancakes in ten minutes that don’t taste flat. It also helps maintain color. Pre-cut potatoes—think frozen fries or hashbrowns—turn gray as starches react with air, but toss them in a tiny bit of this additive, and the spuds stay golden. This keeps packaged potato products looking like they should, saving grocery stores a ton of waste.
Helping Fish Stay Fresh
Walk down the canned fish aisle. Disodium Dihydrogen Pyrophosphate sneaks into many labels. Seafood processors add it to hold color in shellfish and fish, making sure canned shrimp look pink and not brown. Without it, shoppers might skip over products that seem off-color, regardless of the actual freshness or taste inside the tin.
Reality of Processed Food
I’ve met plenty of shoppers leery about long chemical names. People ask, “Why not just use real food?” Fact is, mass production changes what’s possible. Foods move from farm to shelf over weeks or months. Longer shelf life lowers prices and keeps pantries fuller. Few want gray French fries or crumbling biscuits. Additives operate in the background, supporting both food safety and appetite appeal.
Safety and Controversy
Disodium Dihydrogen Pyrophosphate carries a thumbs-up from food safety agencies, used within clear limits. That doesn’t mean conversations about food safety stop. There’s real concern over eating too many phosphates. Studies find that high phosphate diets might link to kidney issues or bone problems, especially for people with kidney disease. The more processed foods we eat, the more of these additives sneak into our meals, and few of us keep track without reading every label.
Better Choices and Next Steps
Real change happens at home and in the industry. At home, cooking from scratch and buying less processed food cuts down on phosphate intake without overthinking it. Companies can look for new ways to solve old problems, like inventing better packaging or using natural acids instead. It’s up to regulators and researchers to make sure these swaps keep food both safe and appealing. Food producers benefit when they pay attention to changing consumer demands. Folks want shorter ingredient lists, and companies making the first move win both trust and business.
Is Disodium Dihydrogen Pyrophosphate safe to eat?
The Additive With a Long Name in Everyday Foods
Disodium dihydrogen pyrophosphate pops up all over the grocery store, hiding behind that chemistry class name. Most people cross paths with it through instant mashed potatoes, frozen seafood, or those chewy baked goods that hold their color a little longer than food used to. When I first started reading labels seriously, I had to stop and look this one up, unsure why something so technical belonged in food rather than a lab.
Why the Food Industry Uses It
As a leavening agent, disodium dihydrogen pyrophosphate makes dough rise evenly and keeps French fries and potato chips from turning gray after cooking. That’s why many commercial bakers and snack manufacturers rely on it. Product appearance still matters, even with all the focus on proteins and “superfoods” these days. I’ve watched chefs use various methods to keep potatoes looking fresh, but the industrial world leans on chemicals for speed and consistency. That’s the trade-off people don’t always realize when picking up a bag of chips at the store.
What Research Shows About Safety
Regulatory bodies, including the U.S. Food and Drug Administration, consider disodium dihydrogen pyrophosphate safe in food at allowable levels. Studies suggest the body processes this phosphate, with most being expelled through the kidneys. Even so, some scientists raise questions about phosphate additives in general. They point to research linking high phosphate intake with potential health risks, especially for folks managing chronic kidney disease. Evidence reviewed by the European Food Safety Authority in 2019 found phosphate additives didn’t pose an immediate threat to healthy people when consumed within recommended limits. For someone like me with an active lifestyle and normal kidney function, eating products with this additive every now and then poses minimal concern.
Concerns With Overuse—Not the Compound Itself
For the average eater, the primary worry comes from eating processed foods constantly, not just one ingredient like disodium dihydrogen pyrophosphate. These foods often add up, and phosphate from many sources can trickle over the limit agencies set without people realizing it. I’ve noticed in my own family—young and old alike—that most of us eat a lot more processed snacks and frozen meals than our grandparents did. Reading about the science is one thing, but seeing how aisles have shifted toward more shelf-stable, ready-to-eat things in the last decade shines another light on the issue.
Ways Forward: What Can Be Done?
Cutting out processed foods completely sounds impossible, especially on a busy schedule. Focusing on whole fruits and vegetables and more home-cooked meals can keep additive intake much lower. If you need packaged food, picking items with shorter ingredient lists works well. I’ve made it a habit to check the nutrition facts, especially the phosphate content, for my parents who have kidney challenges. Companies can help too: clear labeling and cutting back on unnecessary additives would help families make more informed choices.
Disodium dihydrogen pyrophosphate isn’t the villain here; it’s more about how modern eating habits have changed. If you keep processed foods as a small part of your diet, this additive doesn’t bring much risk for most people. For those managing kidney problems or other health issues, a conversation with a dietitian before making changes always makes things smoother.
Does Disodium Dihydrogen Pyrophosphate contain allergens?
Understanding Disodium Dihydrogen Pyrophosphate
Disodium dihydrogen pyrophosphate often pops up on ingredient labels of processed foods like instant mashed potatoes, tortillas, and some baked goods. Those long chemical names look intimidating, and I’ve heard plenty of questions from friends and family about what these things mean for people with food allergies. I dug into this topic myself after a neighbor’s child had a nut allergy scare, even though the snack that caused it didn’t list any obvious triggers. Their concern got me thinking more about food labels and what’s really in those hard-to-pronounce additives.
Looking for Allergens in Disodium Dihydrogen Pyrophosphate
This ingredient doesn’t come from nuts, dairy, soy, wheat, or the other top eight allergens flagged by the FDA. Instead, manufacturers produce it by combining phosphoric acid and sodium carbonate, both of which come from minerals. Chemically, it doesn’t have proteins that the immune system would attack the way it does with peanuts or eggs. For people who need to read every label twice—whether due to a personal allergy or someone they care about—this makes a difference.
How Production Impacts Food Safety
Many food-grade chemicals could pick up traces of allergens during manufacturing, but that risk depends on the facility’s cleaning practices and what else gets made there. Manufacturers regularly issue “may contain” statements if there’s a chance of cross-contact. Companies in the United States and Europe warn consumers about even the possibility of allergens in food, because it’s a safety and regulatory issue, not just a customer service matter. Watching how these alerts show up on labels matters as much as reading the ingredient list itself.
Regulation and Ingredient Transparency
Food safety authorities in the US, Canada, and EU agencies require clear labeling for nine major allergens. Disodium dihydrogen pyrophosphate hasn’t made that list. It lands in processed foods mostly for its technical benefits, like helping dough rise or keeping potatoes from going gray. The FDA tests the safety of these additives before they ever show up on a supermarket shelf. Any new allergic reactions to a food additive bring an immediate review, and authorities update recommendations if science shifts. So far, there’s little evidence that this additive hurts people with allergies, outside of extremely rare cases of sensitivity or phosphate metabolism disorders.
Personal Choices and Potential Solutions for Sensitive Consumers
Folks who struggle with multiple allergies or have a child with extra-vigilant dietary needs know that complications still exist. Cross-contamination can creep in for any packaged item, not just the ones with obviously risky ingredients. I tell my friends to contact manufacturers if they can’t tell from the packaging whether a product was handled in a safe facility. More companies now use QR codes or call center numbers for these questions, but it often takes some persistence. The best answers usually come straight from the horse’s mouth, not just the fine print on the box.
Supporting Safe Food Choices Across the Board
Pushback from consumers has shaped better transparency for food additives across the industry. Groups like FARE (Food Allergy Research & Education) and Anaphylaxis Campaign keep pushing for better labeling and more research. Their advocacy matters because food supply chains keep getting more complicated. Every shopper who picks up the phone or writes an email about unclear allergen risks contributes to safer, more honest packaging for everyone else down the line. People with severe allergies benefit most from this vigilance, but these improvements help every family make smarter decisions at the grocery store.
What foods commonly contain Disodium Dihydrogen Pyrophosphate?
Understanding What It Is
Disodium dihydrogen pyrophosphate—long name, short nickname: SAPP—plays a big role in processed food. I stumbled onto this additive once I started reading ingredient lists a bit closer after a bad reaction to a frozen dinner. Curiosity took over. The more I learned, the more I realized how widespread SAPP really is, especially in foods that promise easy prep and an even, tasty result every time.
Baking Mixes and Convenience Breads
My old box of pancake mix didn’t just have flour and sugar. Disodium dihydrogen pyrophosphate jumped out as part of the “leavening” blend. This additive helps baking powders create carbon dioxide, so pancakes rise fluffy and baked goods don’t fall flat, even if they sit on a supermarket shelf for months. Mass-market tortillas, frozen biscuits, and a lot of store-brand muffins also list SAPP high up—food companies count on it to give that reliable texture people expect.
Potato Products: From Fries to Instant Mash
Frozen potato wedges, hash browns, and instant mashed potatoes often get a dose of SAPP. The additive stops spuds from turning gray after processing. When I worked a summer in a cafeteria, I noticed the bulk bags of potato flakes arrived with a sharp, slightly metallic smell, and as it turns out, that’s partly thanks to SAPP as a color protector. Without it, fries and chips risk taking on an unappetizing brown or gray tinge before even reaching your fryer or oven.
Deli Meats and Processed Seafood
Sliced turkey, ham, and even canned crab meat frequently list SAPP as a stabilizer. It helps keep colors from fading and reduces water loss, which makes cold cuts look fresher for longer. Many brands of imitation crab, fish sticks, and even some frozen shrimp rely on it. Reading the fine print on those value packs, it hit me how common SAPP really is in anything that’s chopped up, ground, and shaped into something convenient. Large food makers rely on these additives to ensure shelf life and reliable taste, batch to batch.
Cake Mixes and Frozen Biscuits
Cake and muffin mixes thrown together at home rarely catch my eye—until I flip the box and spot SAPP. The additive acts fast, reacting when liquid hits powder, giving instant rise. It’s a staple in any prepacked baking mix on big-box grocery shelves. Frozen canned biscuits—the ones you whack on the edge of the counter to open—also use SAPP so every batch will rise right, making them a Sunday morning favorite for busy families.
Snacks, Crackers, and Beyond
Snack crackers, ready-made pizza crusts, and some Asian-style noodles also use SAPP in small amounts. It’s common in anything that needs to be evenly cooked and have reliable snap or rise. Sometimes, branded veggie chips make use of it for both color and texture. On a busy week, looking to fill the snack cupboard, it’s clear how processed foods rely on these ingredients for their consistency—it’s a big part of why they always taste the same, no matter when or where you buy them.
Why Bother Checking?
Reading labels takes time, but it gave me choices. Folks dealing with phosphorus sensitivity or kidney health issues need to keep an eye out for ingredients like SAPP. Limiting these foods isn’t always easy, but fresh-cooked meals and simple ingredient lists cut down on additives like this one. Brands shifting to fewer and simpler ingredients offer a real choice. Pressure from informed shoppers can move the needle toward healthier processed food—not just for nutrition, but to limit additives like SAPP that fly under the radar in so many staple items.
Are there any side effects of consuming Disodium Dihydrogen Pyrophosphate?
What Disodium Dihydrogen Pyrophosphate Actually Does
Disodium dihydrogen pyrophosphate pops up in a lot of packaged foods, especially ones like instant potatoes, baking mixes, and some processed meats. Food companies use it to help control leavening and keep certain foods from turning brown too fast. My own pantry has seen its fair share of pancake mixes listing this chemical near the top. The food world calls it “E450” as a shorthand, but the label hardly explains what it means for actual health.
Possible Side Effects: Scientific and Real-World Perspective
Over the past few years, I have noticed more people asking about food additives and what they really do beyond preserving shelf life. With disodium dihydrogen pyrophosphate, most health authorities such as the European Food Safety Authority (EFSA) and the US Food and Drug Administration (FDA) allow it for use within specific limits. Excessive phosphate intake, though, has a track record for raising some red flags.
Researchers link high phosphate diets to mineral imbalances in the body. Too much phosphate can mess with calcium absorption, potentially putting stress on the kidneys. Someone with chronic kidney problems has to pay even more attention. The body, when it faces a steady stream of added phosphates from foods, can’t always filter this load out efficiently. A study published in Journal of the American Society of Nephrology underscored this risk. Phosphates from additives absorb more readily than those found in natural foods, putting extra pressure on already compromised kidneys.
Otherwise healthy adults, on normal diets, usually don’t reach those dangerous levels if they eat a balanced range of foods. Problems crop up most for folks already at risk—people with kidney issues, those with heart problems, and sometimes children with rare metabolic conditions.
Symptoms and What to Watch Out For
Most people probably won’t notice anything out of the ordinary from a snack or a pancake made with this ingredient. Where issues start to show, they often look like muscle cramps, tingling, or consistent digestive problems. Rare allergic reactions have also been reported, including skin rashes or discomfort after eating heavily processed foods.
I’ve talked to several nutritionists who echo the advice: if you have a reason to track your phosphorus intake, reading labels isn’t optional. Too much pyrophosphate in the grocery cart week after week isn’t a silent habit. It can add up, slow and steady.
What Can Be Done: Shopping and Eating Choices
The simplest fix comes from paying attention to how often convenience foods land on the plate. Shifting toward homemade meals gives more control. Egg, baking powder, lemon juice—these basics handle leavening and color preservation in the kitchen just fine, they don’t need a chemical boost.
If health authorities ever dial back the acceptable daily intake, it won’t be because they suddenly found new chemistry, but because more health data tipped the scale. Until that happens, moderation stays smart. Food labeling isn’t just jargon for experts. It’s a toolkit—useful for anyone managing kidney disease or sticking to a low-phosphorus diet.
For those who already live in the grocery aisles reading everything, asking food producers to use cleaner, simpler ingredients keeps pressure on the system. The more people question what goes into their food, the more likely industry will look for ways to meet that need.
| Names | |
| Preferred IUPAC name | disodium dihydroxidooxidodiphosphanium |
| Other names |
Sodium acid pyrophosphate SAPP Disodium pyrophosphate Disodium diphosphate E450(i) |
| Pronunciation | /daɪˈsoʊdiəm daɪhaɪˈdrɒdʒən paɪroʊˈfɒsfeɪt/ |
| Preferred IUPAC name | disodium dihydroxidooxooxidophosphorane(3-)oxidophosphoric acid |
| Other names |
Sodium acid pyrophosphate SAPP Disodium pyrophosphate |
| Pronunciation | /daɪˈsoʊdiəm daɪhaɪˈdrɑdʒən paɪroʊˈfɒsfeɪt/ |
| Identifiers | |
| CAS Number | 7758-16-9 |
| Beilstein Reference | 3529926 |
| ChEBI | CHEBI:63137 |
| ChEMBL | CHEMBL1201610 |
| ChemSpider | 15353 |
| DrugBank | DB13922 |
| ECHA InfoCard | 03c40ba0-08a2-4768-8e54-23f8f8e057e7 |
| EC Number | 231-835-0 |
| Gmelin Reference | 77484 |
| KEGG | C19159 |
| MeSH | D000934 |
| PubChem CID | 24857 |
| RTECS number | UY9100000 |
| UNII | 18W8V6N19W |
| UN number | UN 3278 |
| CompTox Dashboard (EPA) | DTXSID1037397 |
| CAS Number | 7758-16-9 |
| Beilstein Reference | 110505 |
| ChEBI | CHEBI:63137 |
| ChEMBL | CHEMBL1201727 |
| ChemSpider | 14515 |
| DrugBank | DB09406 |
| ECHA InfoCard | 05b955dc-8c3c-4c3f-90a4-9ebd88a9cab4 |
| EC Number | 231-835-0 |
| Gmelin Reference | 65394 |
| KEGG | C01280 |
| MeSH | D000926 |
| PubChem CID | 24808 |
| RTECS number | UW9175000 |
| UNII | 18K5M087XP |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID2020638 |
| Properties | |
| Chemical formula | Na2H2P2O7 |
| Molar mass | 221.94 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.86 g/cm³ |
| Solubility in water | Soluble |
| log P | -4.71 |
| Acidity (pKa) | 1.0 |
| Basicity (pKb) | 1.0 |
| Magnetic susceptibility (χ) | -54.0e-6 cm³/mol |
| Dipole moment | 0 D |
| Chemical formula | Na2H2P2O7 |
| Molar mass | 221.94 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.86 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.3 |
| Acidity (pKa) | 1.0 (first), 7.2 (second) |
| Basicity (pKb) | 1.0 |
| Magnetic susceptibility (χ) | `-52.0e-6 cm³/mol` |
| Dipole moment | 4.8 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −1534.7 kJ/mol |
| Std molar entropy (S⦵298) | 157 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2046 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1894 kJ/mol |
| Pharmacology | |
| ATC code | E450 |
| ATC code | A01AD10 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation |
| GHS labelling | GHS07, Warning, H315, H319, H335 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P301+P312 |
| NFPA 704 (fire diamond) | 1-0-1 |
| Lethal dose or concentration | LD50 Rat oral 2,500 mg/kg |
| LD50 (median dose) | > 300 mg/kg (rat, oral) |
| NIOSH | WF8750000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.75 mg/m³ |
| IDLH (Immediate danger) | Not listed |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 oral rat 2650 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2650 mg/kg |
| NIOSH | WF8750000 |
| PEL (Permissible) | 15 mg/m3 (total dust), 5 mg/m3 (respirable fraction) |
| REL (Recommended) | 0.7 mg/m³ |
| Related compounds | |
| Related compounds |
Tetrasodium pyrophosphate Monosodium phosphate Disodium phosphate Sodium acid pyrophosphate Sodium trimetaphosphate Sodium hexametaphosphate |
| Related compounds |
Sodium acid pyrophosphate Tetrasodium pyrophosphate Monosodium phosphate Disodium phosphate Sodium tripolyphosphate |

