Dipotassium Hydrogen Phosphate: A Down-to-Earth Look
Historical Development
Dipotassium hydrogen phosphate has earned its place in industry and research because users across generations spotted its value early on. Back in the nineteenth century, chemists experimented with simple phosphate salts and discovered their potential for agriculture and food preservation. Dipotassium hydrogen phosphate didn’t just emerge from a vacuum; it evolved out of trial-and-error and the search for more effective fertilizers that didn’t waste precious natural phosphate deposits. As the global demand for food and efficient processing grew, so did the need for stable, water-soluble phosphates. Factories figured out how to synthesize this compound in bulk by neutralizing phosphoric acid with potassium hydroxide. The process simplified operations for companies eager to feed a growing world and preserve food for storage and shipping long before refrigeration became common.
Product Overview
Today, dipotassium hydrogen phosphate appears in food, water treatment, fertilizer production, and even medicine. It shows up on labels in foods as E340(ii) or as a buffering agent to help baked goods rise properly and keep flavors from shifting over time. The technical world turns to this salt because it delivers phosphate and potassium in ratios easy for plants and humans to use. Technicians and food scientists prefer it since it mixes readily with water and doesn’t leave behind many unwanted byproducts. Having worked in chemistry labs myself, I can say that dipotassium hydrogen phosphate is often the go-to choice for preparing buffer solutions, which are crucial for maintaining consistent pH in experiments, water analysis, and laboratory-scale fermentation. Without reliable buffer systems, many processes in dairy research, enzyme manufacture, and antibiotic production would flounder under uncontrolled acidity.
Physical & Chemical Properties
The substance comes as a white or almost white crystalline powder, easy to handle and not prone to clumping if kept dry. It has no real odor, which means technicians keep their noses clear of unpleasant fumes when measuring out hundreds of grams at a time. Its melting point is high enough to remain solid in all normal storage conditions, rarely attracting water if kept sealed. Dissolving smoothly in water, it splits into potassium and phosphate ions—both known nutrients for plants, animals, and people. Its molecular formula stands as K2HPO4, a staple in ingredient and reagent catalogs around the world. You won’t catch the powder fizzing or reacting on its own since it stays stable unless exposed to strong acids or heat. Dense enough to efficiently store in bins or barrels, dipotassium hydrogen phosphate offers manufacturers predictability batch after batch.
Technical Specifications & Labeling
Quality control teams lean heavily on standards like FCC, USP, and various ISO documents that demand narrow ranges for purity, heavy metal limits, and water content. Typical labels feature the chemical formula, grade (food, analytical, or technical), and lot number for quality tracing. The clarity in labeling supports consumer safety and helps pinpoint issues if a problem crops up in the supply chain. Safety data sheets lay out dust-handling advice, grade identification, and emergency guidelines—essentials for anyone working in industrial kitchens, labs, or blending facilities. For companies exporting food additives, there’s considerable paperwork to prove the material matches national and international requirements—anything less risks shipment rejections or recalls.
Preparation Method
Chemists crank out dipotassium hydrogen phosphate by adding potassium hydroxide to phosphoric acid in measured steps, controlling mixing speed and temperature to avoid splashing and overheating. The process takes place in industrial stainless-steel mixers or glass-lined reactors, where operators weigh reactants with precision balances and monitor pH until they hit the target range. From there, water may get partially evaporated to crystalize the salt, followed by filtration and drying, often in vacuum ovens to prevent water pickup. The solid gets milled if it clumps, then checked for moisture. My time in a production facility taught me how small shifts in temperature or mixing can ruin batch consistency—good records and experienced operators make a world of difference.
Chemical Reactions & Modifications
The flexibility of dipotassium hydrogen phosphate comes from its interactions with acids, bases, and metals. Mix it with strong acid, and it shifts to potassium dihydrogen phosphate or orthophosphoric acid. Combine it with calcium ions, and there’s precipitation of calcium phosphate—one basis for plant nutrient mixes. In high school chemistry lessons, you learn about buffer solutions and pH: dipotassium hydrogen phosphate is at the heart of many such mixtures. It even participates in phosphorylation reactions in biotechnology, helping to drive or regulate processes involving DNA, proteins, and other vital biomolecules.
Synonyms & Product Names
Look at ingredient lists, labels, or catalogs and you see this compound hiding under names like DKP, potassium phosphate dibasic, or simply E340(ii) in food. Some call it dipotassium phosphate or dibasic potassium phosphate. Pharmaceutical suppliers present it with United States Pharmacopeia (USP) or European Pharmacopoeia (Ph. Eur.) grades to distinguish intended uses. People who skip Cas numbers or technical codes sometimes miss that all these terms point to nearly the same product—seeing these alternate names prevents buying the wrong material for food, lab, or cultivation.
Safety & Operational Standards
Handling this salt usually ranks low on the hazard scale if you treat it with respect. Wear gloves and dust masks in industrial settings; no one likes dry skin or an itchy throat from airborne particles. Storage stays simple—keep containers sealed, avoid water contamination, mark contents clearly, and check for caking now and then. Safety teams rely on Material Safety Data Sheets (MSDS) for advice about fire, first aid, and environmental disposal. In the event of an accidental spill, sweep up the powder and rinse the area down with one eye on local waste regulations. No major fire risks mean fire crews keep calm, focusing just on basic precautions.
Application Area
Dipotassium hydrogen phosphate shows up all over the map. Agronomy teams use it as a fertilizer to boost phosphate and potassium in fields, supporting strong root growth in crops from corn to potatoes. Food makers toss it into processed cheese to keep texture smooth and into instant puddings to hold thickness. In water treatment, it acts as a corrosion inhibitor for pipes and water heaters; utilities add it to keep minerals from eating away plumbing. Scientists make buffer solutions for everything from DNA extractions to brewing, relying on its reliable pH adjustment. Pharmaceutical developers include it in some IV fluids and nutrition formulations, since it’s gentler on veins than sodium-based salts. Even animal feed producers seek its nutrient boost, knowing that strong livestock need consistent, bioavailable minerals.
Research & Development
Current research keeps unlocking new ways to employ dipotassium hydrogen phosphate. Controlled-release fertilizers draw on its properties to smooth out nutrient supply in greenhouses and large farms. Lab teams explore its role in battery electrolytes and as a medium for cultivating high-value enzymes or pharmaceuticals in bioreactors. I remember test-driving a custom buffer for enzyme stabilization—tweaking the salt ratio yielded improved stability and made the whole process less sensitive to room temperature shifts. Newer projects explore green synthesis pathways using waste phosphate or alternative alkaline sources to lower the environmental footprint of production.
Toxicity Research
Dipotassium hydrogen phosphate scores low on acute toxicity ratings, supported by generations of animal feeding experiments and food safety testing. Too much, though, can throw off electrolyte balance in people with kidney trouble—the same as other phosphate or potassium salts. Chronic exposure studies show mostly mild symptoms at realistic doses, though regulatory groups warn against inhaling fine powders over long periods. In aquatic systems, excess phosphate can spur algae blooms, so waste from manufacture or agriculture demands tight controls. Food safety agencies regularly review new data and update daily intake guidelines to match evolving nutritional and environmental realities.
Future Prospects
As food, fertilizer, and biotech producers look for safer, more sustainable additives, dipotassium hydrogen phosphate holds a strong spot in supply chains. Next-generation farming techniques, such as precision dose application and hydroponics, drive demand for affordable, easily-measured mineral salts. Environmental scientists investigate ways to recover phosphate from wastewater and reuse it as input for this and related compounds, closing the loop on vital mineral cycles. Lab automation and remote monitoring will probably require buffer systems just as reliable as those used today, if not more finely tuned. People want food that lasts, medicines that work safely, and farms that don’t waste nutrients—the legacy and flexibility of dipotassium hydrogen phosphate make sure it will keep serving those goals, provided we keep innovation and safety front and center.
What is Dipotassium Hydrogen Phosphate used for?
From Science Lab to Kitchen Shelf
Dipotassium hydrogen phosphate sounds like something out of a chemistry book, and technically, it is. Still, people put it to work almost everywhere: in food, farming, health, and even in the classroom. This salt carries a mouthful of a name, but it packs a load of value in some unexpected places.
Why Food Industry Trusts It
Walk through any supermarket and you'll spot countless packaged foods. Many brands rely on dipotassium hydrogen phosphate as a food additive. It’s a stabilizer. Take powdered creamers: nobody wants their morning coffee clumping up or separating. Adding this compound keeps everything nice and smooth. Soft drinks and some processed meats use it to maintain an even texture, giving consumers confidence that the product will taste and look consistent every time.
Cheesemaking benefits too. Certain cheeses need just the right pH to get the right flavor and texture. Companies use dipotassium hydrogen phosphate to make this happen reliably. Reliable results are gold in food production—taste and texture keep repeat customers coming back.
A Helping Hand in Healthcare
This compound isn’t just about taste and appearance. Hospitals and clinics use it as a phosphate source in IV nutrition. In cases where patients can’t eat or drink—maybe after surgery or during a serious illness—doctors rely on it to help keep electrolyte levels in check. Balanced electrolytes mean nerves keep firing and muscles keep working. Skimping on electrolytes can lead to dangerous complications. That’s a lesson many have learned firsthand in clinical care: reliable sources can literally save lives.
Some pharmaceuticals include this compound to help the body absorb medication or to maintain safe pH levels in the drug itself.
Helping Plants Grow
Gardeners and farmers know that plants get hungry just like people. Crops need phosphorus and potassium for strong roots and big yields. Spread a little dipotassium hydrogen phosphate on the fields, and you deliver these nutrients right where they’re needed. Years ago, I spent a summer working at a greenhouse. We mixed nutrients with precision, aiming for the perfect growth conditions. Skipping this step meant weak stems and pale leaves, a sure sign the plants missed out on key minerals. Soil and water conditions change from place to place, so knowing exactly what goes into the soil matters a lot for farm output.
Classroom Science and Beyond
Teachers and students run experiments every day that need clear, predictable results. Science classrooms use dipotassium hydrogen phosphate for its buffering power. It helps keep acidity in check, making experiments repeatable and safe. As someone who spent hours in high school chemistry, trust me: confusion over experiment results grows fast when ingredients don’t behave the way you expect. Using well-tested chemicals like this one gives students reliable outcomes to learn from.
Watchouts and Future Directions
Dipotassium hydrogen phosphate plays a useful role, but like salt or sugar, too much of it can create trouble. In food, regulatory agencies limit the dose so diets don’t tip into risky territory. High phosphate intake links to heart and kidney problems, especially where other health issues exist. Farmers also try to balance use—runoff from over-fertilized fields can hit water quality downstream, causing algae blooms or harming aquatic life. Smarter application, soil testing, and following evidence-backed guidelines keep these risks lower.
Industry professionals, farmers, and health experts keep researching better, safer ways to use this ingredient. Food makers test new blends to lower dependency. Medical teams look for improved delivery methods. Sustainable farming practices shift the focus to long-term soil health. Everyone along the way learns from practical experience and scientific evidence.
Is Dipotassium Hydrogen Phosphate safe for consumption?
Understanding Dipotassium Hydrogen Phosphate
Dipotassium hydrogen phosphate shows up as a white, odorless powder in lots of packaged foods, energy drinks, meal replacements, and even some baked goods. On labels, it often hides under the names “potassium phosphate dibasic” or E340(ii). This compound works as a buffering agent, stabilizer, and sometimes as a thickening agent. Its main role: keeping acidity levels in check to help preserve flavor, texture, and shelf life. In the food industry, solutions like this keep things consistent and safe. The U.S. Food and Drug Administration (FDA) classifies it as “Generally Recognized As Safe” (GRAS), meaning experts have seen enough evidence to trust its safety in normal food quantities.
What Science and Regulation Say
Decades of studies have drilled down on food phosphates, checking for toxicity and side effects. At doses found in food, evidence shows no direct harm to healthy individuals. The European Food Safety Authority (EFSA) evaluated phosphate additives, including dipotassium hydrogen phosphate, and set recommended daily intakes based on extensive animal and human data. Going far beyond these safe limits—like extremely high doses in supplements—can cause issues, especially for people with kidney disease, since kidneys clear excess phosphate from the blood. Too much phosphate can imbalance calcium, putting stress on bones and the cardiovascular system. For most people though, food sources don’t come close to these high-risk levels.
Where Personal Experience Meets Food Safety
People often worry when they see chemical-sounding ingredients on labels. I remember the first time I baked bread and saw all the ingredient names in some commercial mixes—it gave me pause until I checked what they actually did. Once I understood that dipotassium hydrogen phosphate keeps bread fluffy by balancing pH, I could see why bakers use it. It’s easy to lump every additive together, but context matters. Many additives, including this one, trace back to common minerals and elements found in soils and plants. The average diet today already includes natural and added phosphates in dairy, meats, nuts, beans, and processed foods. Eliminating them from all foods would mean drastically changing how foods are made, and probably giving up affordable staples that people rely on.
Potential Issues and Practical Solutions
Research points to some groups who should stay alert. People with chronic kidney disease need to limit phosphate intake, since weak kidneys struggle to process and clear out excess. That means doctors will recommend reading labels not just for foods with dipotassium hydrogen phosphate but for all phosphate additives. Labels in the US and European Union list this compound, but keeping the type and amount of added phosphate clear could go further towards helping people track their intake. Educating consumers through health care providers and public initiatives also plays a big part—clearer information often changes food choices more than fear-based headlines do. Food producers and regulators can test new formulations, using fewer additives without sacrificing quality where possible.
Building Trust in the Food Supply
Many experts advocate for a balanced approach. Calling out every additive as harmful does not reflect the evidence. Instead, steady, routine review of phosphate intake in national nutrition surveys can keep food policies effective and up to date. Dipotassium hydrogen phosphate has a history of safe use in regulated quantities. For those with no medical reason to avoid it, concern tends to overshadow actual risk. Real trust comes from transparency, ongoing scientific scrutiny, and the freedom for informed decisions at the grocery store.
What is the chemical formula of Dipotassium Hydrogen Phosphate?
The Basics Every Lab Tech Recognizes
Ask any chemistry student working late in a college lab about dipotassium hydrogen phosphate and you’ll probably get a quick answer: K2HPO4. This formula pops up on many reagent shelves, slipping into routine buffer recipes or fertilizer blends. The formula itself says a lot—two potassium atoms paired with a hydrogen phosphate ion. It’s all about the chemistry of salts made from a weak acid (phosphoric acid) meeting up with a strong base (potassium hydroxide).
Plenty of labs rely on this compound for its predictable performance. We mix it into buffers and count on it to help maintain a steady pH. In plant nutrition, it delivers an ample dose of potassium and phosphorus, two elements plants crave for root growth and fruit development. Factories turn to it as a safe food additive, marked as E340(ii), keeping foods stable and shelf-ready.
Understanding Why It Matters
The importance of dipotassium hydrogen phosphate isn’t only tied to what it does in the lab or field. It’s the reliability and safety profile that matter for both users and consumers. The European Food Safety Authority and the FDA both keep an eye on how much is getting added to food, keeping daily intake under control. People deserve peace of mind when they grab a box of cereal or use a sports drink, and regulations make this possible.
Beyond safety checks, the role of K2HPO4 in sustainable agriculture stands out. Commercial growers hunt for alternatives to keep their crops healthy without damaging the soil or nearby waterways. Responsible use means getting nutrients to plants without overloading the land and causing runoff problems. Fertilizer guidelines and careful monitoring help keep the impact in check.
Challenges and Common-Sense Solutions
Some folks in the farming community worry about overuse. Add too much phosphate to soil, and problems crop up in freshwater lakes and rivers, sometimes fueling harmful algae blooms. Dealing with this means getting smart about fertilizer application. Soil testing can avoid guesswork, dialing in exactly how much dipotassium hydrogen phosphate is needed. Tailored schedules give plants enough nutrients without overshooting and wasting resources.
Education takes the lead here. Extension agencies and local co-ops teach growers how to read soil reports, weigh options, and avoid excess. Workshops about potassium and phosphorus cycling have picked up in popularity, especially in regions where water quality matters to both wildlife and public health.
On the food side, dietitians warn about phosphorus additives in packed foods, especially for people with kidney problems. Consumers pushing for clearer food labels make a big difference—manufacturers have begun disclosing phosphate ingredients more plainly on packages. This helps shoppers make informed decisions about what they’re putting into their bodies.
Real World Advice and Long-Term Thinking
Chemists, food producers, and farmers all keep K2HPO4 in their toolkit. Choosing to use it calls for a little balance: giving crops a boost, extending product shelf life, or keeping a buffer solution right on target. Taking time to get educated about phosphate management helps prevent headaches and protects the things we all care about—health, clean water, and the land we live on. If you’re new to its uses, reading up and asking questions always pays off.
How should Dipotassium Hydrogen Phosphate be stored?
What Matters Most in Storage
Every lab and factory I have ever worked in has one rule above all: keep chemicals safe, dry, and undisturbed. Dipotassium hydrogen phosphate has earned its place in classrooms, production lines, and research labs for a reason. But too many people treat chemicals like blocks in a warehouse. I’ve seen time and again how mishandling turns a perfectly good product into wasted money and sometimes even a safety risk.
Moisture Is the Real Enemy
Check any material safety data sheet and you’ll see the same advice: keep things dry. This potassium salt pulls water straight from the air, so it clumps, cakes, or shifts from powder to paste before you know it. I once tried using an old bag that looked fine from the outside, but the whole bottom half had turned solid. Humidity sneaks in from open windows or even from a loose cap. Dry, cool storage works—desiccators, tight-sealing containers, and a shelf that’s far from heat sources.
Temperature Spikes Cause Problems
Storing dipotassium hydrogen phosphate at room temperature makes sense, but I have seen people stick it next to a furnace just to ‘keep things out of the way’. If you leave it too close to heat, the powder can degrade. Small temperature swings shorten shelf life and sometimes knock purity right off spec. Ideally, tuck it away somewhere that feels comfortable for people—proof enough that it will keep its form for months at a time. Extreme cold isn’t a friend, either, because condensation will join the party as soon as you take the bottle out and set it on a counter out in the open.
Keep It Honest and Labeled
I remember a day in the stockroom when someone grabbed the wrong jar and added potassium salt to a solution by mistake because the label had faded. Clear, legible labeling saves more headaches than any other detail. Write the opening date and always use up the oldest material first. Faded labels, missing caps, or half-used jars leave a trail for confusion, so grab a marker and tape, and keep every jar ready for the next round of experiments or production runs.
Protect It from Contamination
Cross-contamination eats away at reliability. Pouring powder straight from the primary jar, pinching some with a dirty spatula, or diving back in with wet hands—these simple habits undo the controlled environment. I recommend portioning out what you need for each session into a separate beaker, then sealing the main batch right away. Use gloves. Keep tools clean. Don’t swap scoops between chemicals. These steps make a direct difference in consistency and safety.
Handle and Dispose Safely
Spills stick around long after the mess looks clean. Dipotassium hydrogen phosphate seems benign, but in the wrong place, it can trigger slippery surfaces and unwanted reactions. Have an absorbent on hand and wipe up any powder or solution that gets loose right away. Waste piles up quickly, so always mark disposal containers and follow local rules for dumping anything with even a trace of chemical residue.
Learning from Mistakes
In my experience, nobody gets chemical storage perfect on the first try. I‘ve learned most of my best tricks from seeing what goes wrong for others and myself. Taking extra time to check the cap, track the shelf life, and keep things clean adds up to longer-lasting supplies and fewer emergency phone calls. Simple attention to details builds safer, more efficient labs and keeps budgets under control. Dipotassium hydrogen phosphate may act like an everyday chemical, but treating it with respect shapes a better work environment for everyone.
What are the potential side effects of using Dipotassium Hydrogen Phosphate?
Common Uses and Exposure
Dipotassium hydrogen phosphate pops up a lot in food and medicine. It stabilizes foods, improves texture, and controls pH. Hospitals sometimes turn to it for people who need more phosphorus or potassium in their diets, especially through IV fluids. It works well as a buffering agent, so it’s easy to find in everything from processed cheese to powdered drinks.
Potential Side Effects: What People Might Notice
Some people run into minor stomach problems after eating foods with this additive. Nausea, diarrhea, and upset stomach land at the top of the list. Not every person will notice these effects, but folks with sensitive stomachs might feel more discomfort. Reported cases link large doses to disruptions in digestion—think bloating or gas—because the extra phosphate pulls water into the gut and changes how things move through the intestines. I’ve seen this play out in a hospital setting with patients who received phosphate-containing IVs and felt a rumbling gut within hours.
Risks from High Doses: Heart, Kidneys and Electrolytes
Issues become more serious for people who use too much, or for those who already struggle with heart or kidney trouble. High phosphate intake over time can throw off balance in the body’s electrolytes. Some people see big jumps in phosphate and potassium blood levels. This causes problems like irregular heartbeats, muscle weakness, or even confusion. For someone with kidney problems, this risk shoots up because their body can’t clear out the extra phosphate. In rare cases, blood phosphate can climb so high that it starts binding with calcium, which leads to muscle cramps, tingling, or hard deposits forming in tissues.
The scientific community tracks these risks carefully. A study published in the journal Nephrology Dialysis Transplantation points out that high phosphorus and potassium both stress the kidneys and heart. Over 10,000 patients in several long-term studies showed a connection between excess phosphate and heart disease, especially in those needing regular dialysis. Another review in the American Journal of Clinical Nutrition raises concerns about food additives like dipotassium hydrogen phosphate making up more and more of people’s daily phosphate intake.
Vulnerable Groups: Not All People React the Same
People with normal kidney function usually handle moderate exposures with no trouble. On the other hand, those living with chronic kidney disease or heart problems deal with extra complications. Children are also more sensitive to changes in electrolyte levels, especially babies getting specialized infant formula with added phosphates. Older adults face more risk because declining kidney function is common and medications may interact with phosphate-containing additives. Healthcare providers keep tabs on labs for these groups for a reason.
Watching Out: Managing Dietary Phosphate
Reading ingredient labels helps spot sources of added phosphates. Keeping an eye on how much phosphate comes in through processed food reduces risk, especially for anyone with kidney or heart problems. Doctors sometimes prescribe phosphate binders or diet changes for those most at risk. I’ve seen families carefully balance convenience foods with homemade meals to control how much their loved one consumes each day.
Paths Forward: Education and Research
Not everyone knows how many processed foods rely on dipotassium hydrogen phosphate and other phosphate salts. Increased education about food additives helps people make choices that match their own health needs. Researchers keep looking for substitutes and studying long-term intake. Regulators have started to require clear labeling and limit the use in certain foods and medical products. These steps let people weigh the potential benefits and drawbacks before choosing products made with this chemical.
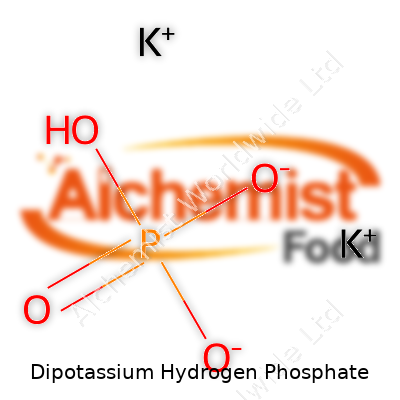
| Names | |
| Preferred IUPAC name | potassium hydrogen phosphate |
| Other names |
Dipotassium phosphate Potassium phosphate dibasic Dibasic potassium phosphate Potassium hydrogen phosphate DKP K2HPO4 |
| Pronunciation | /daɪ.pəˈtæs.i.əm ˈhaɪ.drə.dʒən ˈfɒs.feɪt/ |
| Preferred IUPAC name | Dipotassium hydrogen phosphate |
| Other names |
Dipotassium phosphate Potassium phosphate dibasic Dibasic potassium phosphate DKP Potassium hydrogen phosphate |
| Pronunciation | /daɪpəˈtæsɪəm haɪˈdrɒdʒən fɒsˌfeɪt/ |
| Identifiers | |
| CAS Number | 7758-11-4 |
| Beilstein Reference | 172142 |
| ChEBI | CHEBI:62952 |
| ChEMBL | CHEMBL1201194 |
| ChemSpider | 16211178 |
| DrugBank | DB09453 |
| ECHA InfoCard | 18b4a658-151c-41da-8d85-cf5d178aec89 |
| EC Number | 231-834-5 |
| Gmelin Reference | 62268 |
| KEGG | C14333 |
| MeSH | Dipotassium Phosphate |
| PubChem CID | 24450 |
| RTECS number | TC6615500 |
| UNII | V4WK8LN1XP |
| UN number | UN9147 |
| CompTox Dashboard (EPA) | DTXSID2020288 |
| CAS Number | 7758-11-4 |
| Beilstein Reference | 3566858 |
| ChEBI | CHEBI:62952 |
| ChEMBL | CHEMBL1201658 |
| ChemSpider | 82775 |
| DrugBank | DB09420 |
| ECHA InfoCard | 100.028.764 |
| EC Number | 231-834-5 |
| Gmelin Reference | 62288 |
| KEGG | C00222 |
| MeSH | Dipotassium Phosphate |
| PubChem CID | 516951 |
| RTECS number | TC6615500 |
| UNII | VZ8Z83I7FU |
| UN number | UN9149 |
| Properties | |
| Chemical formula | K2HPO4 |
| Molar mass | 174.18 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 2.44 g/cm³ |
| Solubility in water | 167 g/100 mL (20 °C) |
| log P | -4.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 2.2 |
| Magnetic susceptibility (χ) | −63.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.333 |
| Dipole moment | 6.0 D |
| Chemical formula | K2HPO4 |
| Molar mass | 174.18 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.44 g/cm³ |
| Solubility in water | 167 g/100 mL (20 °C) |
| log P | -0.8 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 6.8 |
| Magnetic susceptibility (χ) | -51.0e-6 cm³/mol |
| Refractive index (nD) | 1.334 (20 °C, 50% aq. sol.) |
| Dipole moment | 6.8 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 205.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1150.46 kJ/mol |
| Std molar entropy (S⦵298) | 228.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1150.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2977 kJ/mol |
| Pharmacology | |
| ATC code | B05XA11 |
| ATC code | B05XA03 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS labelling: `"Signal word: Warning; Hazard statements: H319 - Causes serious eye irritation; Pictograms: GHS07 (Exclamation mark)."` |
| Pictograms | GHS07,GHS09 |
| Hazard statements | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Precautionary statements | Store in a dry place. Store in a closed container. Dispose of contents/container in accordance with local/regional/national/international regulations. |
| NFPA 704 (fire diamond) | 0-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 5,000 mg/kg |
| LD50 (median dose) | > 4,640 mg/kg (Rat, oral) |
| NIOSH | WH7440000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | Not listed |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. |
| Precautionary statements | Store in a dry place. Store in a closed container. Dispose of contents/container in accordance with local/regional/national/international regulations. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Explosive limits: Non-explosive |
| Lethal dose or concentration | LD50 (oral, rat): 4,180 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 5,000 mg/kg |
| NIOSH | GB243-04 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/kg bw |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Monopotassium phosphate Tripotassium phosphate Disodium phosphate Dipotassium phosphate Potassium dihydrogen phosphate |
| Related compounds |
Monopotassium phosphate Tripotassium phosphate Potassium dihydrogen phosphate Sodium phosphate Potassium phosphate |

