Diammonium Hydrogen Phosphate: Origins, Uses, and the Science Behind It
Historical Development
Diammonium hydrogen phosphate, often found under abbreviations like DAP, first drew interest during early advances in industrial chemistry through the late 19th and early 20th centuries. Growing demand for fertilizers drove research teams to isolate effective compounds that would both provide key nutrients and dissolve well in water. Chemists in Europe and the United States discovered the simple reaction between ammonia and phosphoric acid could produce a stable salt that solved key limitations with single-nutrient phosphorus sources. Attention quickly turned to mass production and DAP became a staple in agricultural supply lines by the middle part of the 20th century. From its historical start in simple factory setups, today’s production relies on tight quality controls and a clear understanding of downstream impacts on soil and water.
Product Overview
Diammonium hydrogen phosphate looks like a white granular or crystalline powder. Farmers, horticulturalists, and chemical industries keep it on hand for its ready solubility and strong nitrogen and phosphorus content. It stands out for its 18-46-0 N-P-K rating—meaning 18% nitrogen and 46% phosphorus pentoxide, with zero potassium, making it a crucial source for two out of the three key macronutrients every farmer tracks. Commercial operations prefer it in bulk for both crop nutrition and as a fire retardant, while food-grade variants find use in yeast nutrition and water treatment. These details matter on the ground—growers often report that DAP gives crops an early-season lift and reliable results across climates, making it a consistent performer in field trials.
Physical & Chemical Properties
This salt appears odorless and stays stable under typical storage, melting only above 155°C with decomposition. DAP dissolves easily in water, splitting into ammonium and phosphate ions. The pH of a typical solution tends to hover near neutral or slightly alkaline, useful in keeping application equipment clean and preventing clogging. In my days working alongside fertilizer blenders, we weighed DAP for its lack of dustiness and free-flowing properties, which sped up mixing and minimized loss on those windy days common to open sheds. It weighs in at a molecular weight of 132.06 g/mol and shows little volatility, so loss post-application stays lower than many comparable phosphate products.
Technical Specifications & Labeling
Label regulations keep everyone from farmers to warehouse crews on the same page. Regulations demand the packaging to show the content of total nitrogen, ammonia nitrogen, and water-soluble phosphorus pentoxide with tight tolerances. For agricultural trade, DAP must meet granule size ranges to avoid dust and misapplication. Packaging often involves moisture-resistant bags with legible hazard statements based on workplace safety guidelines. Every bulk order ships with a certificate indicating country of origin, batch analysis, and guaranteed minimums for macronutrients. This level of scrutiny remains a must as contamination, or misstatements cost both trust and crop yield.
Preparation Method
Industrial manufacture of DAP starts with concentrated phosphoric acid reacted with ammonia gas under controlled temperatures. The recipe matters—a slight excess of ammonia ensures ammonium ions bond with both hydrogen phosphate and to some extent convert some phosphoric acid to monoammonium phosphate if pH stumbles. The resulting slurry cools to yield fine crystals, filtered and dried to commercial standards. Recovery and reuse of byproducts elevate efficiency, cutting waste streams. In practice, most plants recycle the cooled mother liquor and trim ammonia emissions using scrubbing or other abatement systems, a testament to tightening environmental oversight in production zones.
Chemical Reactions & Modifications
Once dissolved in water, DAP undergoes dissociation, freeing up ammonium (NH4+) and hydrogen phosphate (HPO42-) ions. This reaction plays a core role in plant nutrition. Soil enzymes convert these into forms available for plant uptake, fueling early root growth and boosting stress tolerance. In lab settings or specialty applications, DAP acts as a precursor for other ammonium phosphates through pH adjustment and secondary ammoniation steps. Blenders sometimes modify DAP with stabilizers to slow ammonia loss in warm climates or to add chelated micronutrients, but pure DAP remains a go-to for its predictability.
Synonyms & Product Names
Beyond its main name, you’ll see diammonium hydrogen phosphate listed as diammonium phosphate, ammonium monohydrogen phosphate, or even DAP. Commercial bags may highlight brand names or simply abbreviate straight to its chemical initials. In global markets, translations or alternative nomenclature can cause confusion, making those batch certifications and technical sheets a vital piece of the trade. Supply catalogs and safety data both avoid ambiguity by listing chemical formula (NH4)2HPO4 alongside trade names.
Safety & Operational Standards
Workers handling DAP in factories or farm settings must follow clear protocols. Eye and skin contact rarely lead to lasting harm but can cause irritation, especially with dust exposure. Respiratory protection cuts down risk in confined or dusty operations. Most large facilities rely on atmospheric testing to keep airborne ammonia and fine particulate below safety triggers, and standardized first aid guidance covers every shop floor. DAP does not burn but can add to flame retardant mixes for forest management and urban fire prevention. Safe storage depends on keeping the product dry and segregated from acidic or alkaline materials that can break it down or prompt unintended release of ammonia vapors.
Application Area
DAP’s primary market sits in agriculture—spread as a base fertilizer for cereals, legumes, vegetables, and fruit plantations, especially where phosphorus-deficient soils limit yields. In controlled amounts, it also functions as a leavening agent in baking, a yeast nutrient in brewing, and a catalyst for certain industrial reactions. Water treatment plants dose it in selective cases to limit lead pipe corrosion. In forest management, fire crews use DAP blends to coat vegetation and slow wildfires. My own experience in rural crop extension visits confirms its refilling demand among growers aiming for feed grain above baseline protein targets.
Research & Development
Even with decades of established practice, research on DAP continues at universities and industry labs. Climate pressures and shifting global phosphate reserves drive interest in improving nutrient use efficiency, especially with growing scrutiny on runoff and water quality. Reports from field trials compare DAP with coated or stabilized phosphates, testing both yield and environmental impact. Researchers also focus on synergistic blends with micronutrients or bio-stimulants for site-specific improvement. Companies invest in process improvements to trap more ammonia and boost the life-cycle sustainability profile of each batch. As demand climbs in regions adopting higher-yield farming, R&D seeks ways to stretch phosphorus through recycling and smarter formulation.
Toxicity Research
Toxicological evaluations find DAP presents minimal acute risk to humans or livestock when handled and applied correctly; oral exposures at very high levels could lead to mild gastrointestinal disturbance due to ammonia liberation, but this sits well above routine accidental doses. Chronic exposure studies, both in animal models and environmental monitoring, mostly link risks to broader nitrogen and phosphate cycles rather than direct toxicity. One concern involves aquatic toxicity when runoff accumulates and disrupts freshwater systems, triggering algal blooms and downstream eutrophication. On farms, management pivots toward reduced-rate application, buffer strips, and timing with plant demand—all to cut nutrient loading in nearby waterways and keep DAP’s environmental footprint center-stage in stewardship talks.
Future Prospects
With the world’s population growth pushing pressure on crop systems and phosphate ore supplies facing geopolitical bottlenecks, DAP’s continued relevance hinges on both supply chain resilience and better agronomic strategies. Advances in slow-release technologies, specialty blends tailored for exhausted or marginal soils, and data-driven precision application find eager adoption across progressive growers. On the sustainability front, moves to complement DAP with organic sources and to reclaim phosphorus from municipal waste streams set the direction for closed-loop approaches. Research into alternative polyphosphates and new ammonium salts may eventually push DAP aside, but for now, it remains the workhorse, spotlighting the balance between yield boost and environmental responsibility.
What is Diammonium Hydrogen Phosphate used for?
Down-to-Earth Uses That Touch Daily Life
Diammonium hydrogen phosphate shows up as a plain-looking white powder, but the jobs it handles reach beyond what most folks expect. Most people know it as a plant booster in the farming world. I’ve seen it stacked high in co-ops out in rural areas, with farmers lining up for their allotment. On the farm, it gives both nitrogen and phosphorus—two things crops crave as they push out of the soil in early spring. A solid start with these nutrients means a bigger yield and stronger plants that can handle tough weather.
This chemical plays another role most people don't see. Bread and baked goods land on supermarket shelves because of leavening agents. Diammonium hydrogen phosphate works quietly in the dough, feeding the yeast. I remember baking bread that struggled to rise, only to learn from a seasoned baker that this ingredient keeps bakery items soft and helps their crust brown just right. It’s not about taste, it’s the chemistry in making food shelf-stable and appealing. The European Food Safety Authority and the U.S. Food and Drug Administration both keep watch on its safe use in food—strict limits matter, especially for kids who snack on baked treats daily.
Fire Prevention and Industrial Lifelines
Outside kitchens and cropland, you’ll spot diammonium hydrogen phosphate snuffed across flames as part of fire-extinguishing powders. I’ve worked on older buildings where insurance required us to install extinguishers filled with dry chemical agents. In a fire emergency, seconds count; this compound’s ability to smother flames and block re-ignition offers real peace of mind. Some households, especially in wildfire-prone regions, keep big drums of the stuff on hand, knowing it can give a survivable edge until fire crews get there.
Look inside factories, and you’ll catch this chemical showing up yet again. Metal finishers swear by it for surface treatment. It cleans, primes, and helps paints or coatings stick without ugly flaking. Engineers in water treatment lean on diammonium hydrogen phosphate for buffer solutions to keep acidity steady in both laboratory and municipal water systems. These behind-the-scenes uses might never get a spotlight, yet they support daily conveniences from clean water to rust-free bolts.
Environmental Care and Cautions
With all its usefulness, there’s a flip side worth real talk. Too much phosphorus dumped into waterways sparks algae blooms that choke lakes and rivers. I remember a summer camping trip at a lake thick with green scum—fishing canceled, beaches closed. That stuck with me. Responsible use starts before application. Farmers follow rules for spreading to make sure rain doesn’t wash it away. Cities invest in water treatment tech so only the right amount stays in tap water and the rest is filtered out.
Simple solutions make the biggest difference—tighter regulation, honest education for anyone handling or selling these products, and supporting research into even safer alternatives. Knowing exactly what’s in fertilizers and food, down to the gram, gives families and growers real control over health and their land. Careful stewardship and clear rules keep both crops and communities safe. Diammonium hydrogen phosphate might seem ordinary, but in practice, it holds together a web of food, safety, and industry that most folks rely on every day.
Is Diammonium Hydrogen Phosphate safe for human consumption?
Understanding What Diammonium Hydrogen Phosphate Is
Diammonium hydrogen phosphate, sometimes called DAP, shows up on ingredient labels in a few places you might not expect. Food producers use it while making yeast-raised bread and in some drinks like root beer. It's also part of the fertilizer industry, which gives some people pause. Not everything found in agriculture belongs in a bakery, and people naturally want to know if DAP crosses some safety line.
Food Additive Rules and Reality
Regulators like the U.S. Food and Drug Administration have set rules about what makes a compound safe to eat. DAP lands on the list of substances recognized as Generally Recognized as Safe (GRAS) when it's used in the right way and amount. In real kitchens and commercial bakeries, people use it in small amounts to help yeast ferment more efficiently or to add a minor source of phosphate. The yeast use it up for fuel, and hardly any ends up in the final product.
That all sounds simple, but it's worth remembering that "safe if used properly" means you shouldn't go overboard. A steady diet loaded with phosphate-rich additives may raise phosphate levels in the body, especially in those with kidney disease or anyone who struggles to clear excess phosphorus. Health organizations, including the European Food Safety Authority, have pointed out that even approved food phosphates shouldn’t be consumed in unlimited quantities. Most people eating a typical diet won't come close to these thresholds, but folks relying on a lot of processed foods might.
The Chemistry and Why People Worry
Seeing a chemical name on an ingredient panel still bothers many people. Diammonium hydrogen phosphate is a salt formed from combining phosphoric acid and ammonia. By itself, ammonia sounds toxic, and concentrated phosphoric acid isn't a household pantry item either. In DAP, those ingredients come together to form a new compound with very different characteristics.
In my own life, I grew up hearing stories about bread baked with “natural” ingredients—meaning anything with a chemical-sounding name went straight into the “avoid” list. That way of thinking doesn't always match the facts. Modern food science has shown that lots of hard-to-pronounce additives are tested and monitored better than the vague “natural” additives from old family cookbooks. DAP doesn't stick around in the food at high levels, and tests haven’t shown it to be especially risky except for the rare few with phosphate sensitivities.
What Safer Choices Might Look Like
For people who want tighter control over what goes in their meals, skipping processed foods helps keep phosphate intake and other additive exposure low. Baking your own bread or cooking from scratch gives peace of mind about every ingredient. Food companies could do better at transparency—listing exactly why something like DAP is in the loaf or drink.
If you have health conditions—especially kidney issues—ask a dietitian or doctor about phosphate additives. Label reading also helps; turning to brands that skip additives can cut overall phosphate exposure. Pushing for clearer labeling laws and more consumer education helps everyone make informed decisions. After all, trust in food safety grows when people feel empowered to understand what's on their label and choose accordingly.
What is the chemical formula of Diammonium Hydrogen Phosphate?
Why Diammonium Hydrogen Phosphate Matters
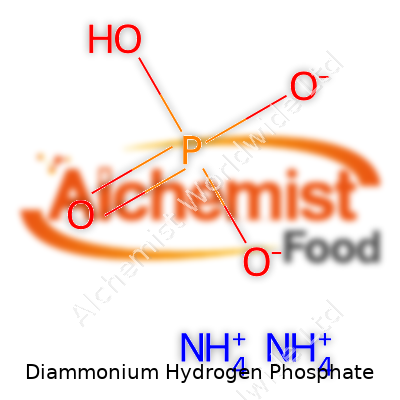
Diammonium hydrogen phosphate is a compound that a lot of people have never thought about, but it's known in classrooms, farms, and even fire safety. The chemical formula for diammonium hydrogen phosphate is (NH4)2HPO4. This formula might look intimidating in print, but breaking it down reveals the essential building blocks: two ammonium ions (NH4+) joined with a hydrogen phosphate ion (HPO42-).
Personal Insight Into Its Use
Back in school, I remember being told to respect the chemistry lab—even the white powders on the shelves that everyone else ignored. Diammonium hydrogen phosphate was a familiar jar for anyone who spent time learning about fertilizers, since it shows up in both science and daily life. Each element in its formula—nitrogen, hydrogen, phosphorus, and oxygen—plays a real, direct role, especially in farming and gardening. Farmers rely on this chemical for better harvests, since it gives crops access to nitrogen and phosphorus, two nutrients that most soils can’t deliver in the quantities that fast-growing plants crave.
Breaking Down the Formula
This compound has two ammonium ions. Those carry the nitrogen. Nitrogen, as anyone who’s tried to grow tomatoes knows, is critical for leaf growth and green color. The hydrogen phosphate part delivers phosphorus and oxygen. That’s the backbone for strong roots and healthy plant development. These elements combine in a way that the plant absorbs easily, turning soil into food. It’s this blend of nitrogen and phosphorus that sets diammonium hydrogen phosphate apart from more basic fertilizing salts.
Industrial and Practical Importance
Walk into a garden center, and you’ll see fertilizers everywhere, often with numbers representing the N-P-K ratio: nitrogen, phosphorus, and potassium. Diammonium hydrogen phosphate usually shows up with a strong N and P number, helping balance soils that might otherwise stunt growth or lead to unhealthy plants. The formula isn’t just important for plant lovers, though. In industry, it also contributes to fire retardants. The chemical’s ability to react in a fire and create a non-flammable barrier makes it useful for producing powders that get packed into fire extinguishers. It’s pretty satisfying to know that the same chemistry that grows beans in a garden also stands between a small kitchen fire and disaster.
Supporting the Evidence With Facts
Plenty of published research shows the effectiveness of diammonium hydrogen phosphate fertilizers in both small gardens and commercial agriculture. According to studies from agricultural extensions, using it can boost crop yield by supplying up to 46% phosphorus pentoxide (P2O5) and 21% nitrogen. Those numbers mean this compound brings both quick and sustained availability of nutrients. Its water solubility is high, so plants get the nutrients they need—not just a slow trickle, but a real burst right when it matters most.
Looking at Solutions and Safer Use
The real challenge comes down to responsible handling and impact on the environment. Overuse of phosphate fertilizers, including diammonium hydrogen phosphate, can lead to runoff problems and algae blooms in rivers and lakes, a tough outcome for both wildlife and people. Expanding soil testing and focusing on the right rate of application for crops keeps food systems working without making water unsafe. Companies also look for ways to regulate the release rate through improved formulation, and more farmers are reaching out for precision agriculture services to match fertilizer use with real plant needs.
Conclusion
Diammonium hydrogen phosphate’s chemical formula, (NH4)2HPO4, isn’t just a string of letters and numbers. Its mix of ammonium and hydrogen phosphate connects classroom science, agriculture, and safety in ways that don’t always get appreciated. With balanced, evidence-backed use, this compound keeps food growing, fires at bay, and science moving forward.
How should Diammonium Hydrogen Phosphate be stored?
Understanding What’s at Stake
Diammonium hydrogen phosphate, or DAP as most folks know it, plays a big role both in agriculture and in industrial settings. Farmers mix it into soil to give crops the phosphorus and nitrogen they crave. Some cities use it to keep their water clean. In the wrong hands—or with sloppy storage—it can cause real trouble. Now, most people see DAP as “just fertilizer,” but mix moisture, heat, and a bit of carelessness, and you have damaged product at best—or a real safety hazard at worst.
Weather isn’t Your Friend
Humidity slowly chips away at stored DAP. If the bag’s left open or the warehouse leaks, you’ll wind up with a clumpy, caked substance. I’ve seen farmers discover their stock has fused into a rock-hard chunk. Not only does that make spreading impossible, it means water has started chemical changes, leaving the material less useful—and sometimes pungent. Damp DAP may create ammonia fumes, which no one wants lingering in a storage shed. Mold finds its way too, another reason to keep things bone-dry.
Choose Storage Spaces with a Farmer’s Eye
Solid walls, a roof in good repair, and above all—a place far away from direct sunlight or heat sources. High temperatures give DAP unnecessary stress, and if you’ve ever opened a bag after it sat near a dryer or a tractor’s exhaust, you’ll know what I mean. Ventilation makes a difference, too. Air movement keeps moisture from hanging around.
Store DAP off the floor on wooden pallets or shelves to keep ground moisture out. Even seasoned pros sometimes forget: concrete sweats, especially through humid summers. Lay down plastic sheeting for peace of mind. If the area floods or sees a big leak, pallets give a bit more time to save inventory.
Don’t Gamble on Compatibility
Never stack DAP near strong acids, alkaline materials, or anything combustible. I’ve talked to more than a few facility managers who thought “it’s just a fertilizer, it’ll be fine.” Then they found themselves dealing with corroded containers or strange smells. Ammonia-based products like DAP just don’t play nice with harsh chemicals. You want your chemicals tagged and separated just like your family keeps cleaning bleach away from vinegar under the sink.
Protect People, Not Just Product
Every workplace has rules on labeling and safety data sheets. It’s not red tape—this stuff works. Clear labels help anyone walking in to know what they’re dealing with. Emergency contacts posted on the wall near your storage area save time if an accident ever happens.
Anyone handling DAP needs gloves, a mask, and good work boots. The powder loves to fly and stick to sweaty hands. Gloves keep skin safe and stop accidental ingestion. Masks help in case the air feels sharp with dust or ammonia. Simple habits stop health problems before they start, especially during the busy season.
Simple Fixes for Everyday Safety
Good storage doesn’t take fancy tools or big budgets. Build a habit: check bags for punctures, sweep up spills right away, and rotate your stock so you always use the oldest bags first. If you spot a leak or strong smell, don’t ignore it—fix the problem or call someone who can.
Storing chemicals like diammonium hydrogen phosphate isn’t glamorous, but it’s necessary work. Ask experienced hands for advice, stay organized, and use your senses. You’ll protect the product, the people, and the land you care about.
Is Diammonium Hydrogen Phosphate environmentally friendly?
Fertilizer: The Double-Edged Sword
Diammonium hydrogen phosphate, known in many places as DAP, pops up in countless farm supply stores and finds a spot in backyard gardens and industrial fields alike. It's a big deal in the world of agriculture because plants grab onto its nitrogen and phosphorus fast. Crops love this stuff, and the yields reflect that love. People like me who’ve tended poor soils appreciate how DAP gives weak plants a kick.
The story gets complicated on the other side of farm boundaries. The nutrients that push crops to grow also run off easily—especially if irrigation or a big downpour comes through. Nitrogen and phosphorus don’t just disappear; they seep into groundwater or slide off into streams and rivers. That triggers algae blooms in lakes and coastlines, choking out fish and turning water foul. Ask anyone living near a green lake in midsummer, and you’ll hear the pain—fishing dries up, swimming becomes risky, and water bills climb as cities scramble for clean supplies.
Production Side Problems
Making DAP isn’t a clean job. Raw phosphate rock and ammonia carry their own environmental loads, including mining scars, air emissions, and chemical waste. Factories pump out greenhouse gases. In my experience visiting a few fertilizer operations, the air smells sharp, and there’s a steady trickle of waste to treat. Sometimes local communities push back, trying to keep their water and soil from picking up heavy metals that come along for the ride.
Soil Health and Farming Practices
It’s tempting to grab a bag of DAP each planting season and call it good, but soil tells a different story over time. Heavy, repeated doses pile up phosphorus, locking out other nutrients and making soil less balanced. Microbes that used to break down organic matter and help roots don’t handle that load well. Years ago, I learned this the hard way—after using DAP season after season, my tomatoes stalled and the soil got hard and pale. Bringing back compost and rotating crops restored what the chemicals had washed out.
Regulation, Alternatives, and Farmer Knowledge
Governments take notice when contamination grows. In places like the European Union, strict rules control fertilizer use, forcing farmers to account for how much runs off their fields. Some regions call for buffer zones near streams or limit applications based on soil tests. That pressure pushes people to consider alternatives: slow-release fertilizers, organic sources like manure or compost, and cover crops that keep nutrients anchored. Some researchers look at biochar and rock phosphate, searching for ways to get nutrients to crops with less spillover.
One root of the problem comes from not knowing the field’s true needs. Soil testing, local weather, and crop choice matter. If farmers can match DAP to what crops and soils really need, waste drops sharply. In my own gardens, things improved after I got the shovel and little test kits out, cutting my fertilizer bags by half and seeing healthier plants all around. Sharing this kind of hands-on knowledge could make a far bigger dent in pollution than any single new product.
Main Takeaways and Looking Forward
People count on DAP because it delivers quick results. The catch is, both the production and the overuse bring harm beyond farm fences. Balancing food needs and environmental care asks for more than switching products or regulations—it calls for grounded advice, better habits, and tech that helps farmers and gardeners work with the land, not just against it.
| Names | |
| Preferred IUPAC name | Diammonium hydrogen phosphate |
| Other names |
Ammonium phosphate dibasic Diammonium phosphate DAP Diammophos DAP fertilizer |
| Pronunciation | /daɪˌæmˈoʊ.ni.əm ˈhaɪ.drə.dʒən fəˈsfeɪt/ |
| Preferred IUPAC name | diammonium hydrogen phosphate |
| Other names |
Diammonium phosphate DAP Ammonium hydrogen phosphate Ammonium phosphate dibasic Ammonium monohydrogen phosphate |
| Pronunciation | /daɪˌæmˈoʊniəm ˈhaɪdrə(d)ʒən fəˈsfeɪt/ |
| Identifiers | |
| CAS Number | 7783-28-0 |
| Beilstein Reference | 1694447 |
| ChEBI | CHEBI:63006 |
| ChEMBL | CHEMBL1201647 |
| ChemSpider | 8848 |
| DrugBank | DB09467 |
| EC Number | 231-987-8 |
| Gmelin Reference | 11370 |
| KEGG | C100028 |
| MeSH | Dihydrogenphosphate, Diammonium |
| PubChem CID | 24540 |
| RTECS number | BQ9625000 |
| UNII | 57U869G6DN |
| UN number | UN2061 |
| CAS Number | 7783-28-0 |
| Beilstein Reference | 1711310 |
| ChEBI | CHEBI:62988 |
| ChEMBL | CHEMBL1201479 |
| ChemSpider | 82667 |
| DrugBank | DB09449 |
| ECHA InfoCard | 03b7e83d-bab1-49bd-99a5-7db6d0217a45 |
| EC Number | 231-987-8 |
| Gmelin Reference | 52941 |
| KEGG | C14120 |
| MeSH | Dihydrogenphosphate, Ammonium |
| PubChem CID | 10361 |
| RTECS number | TB6125000 |
| UNII | FXR6Q8X0KN |
| UN number | UN2061 |
| Properties | |
| Chemical formula | (NH4)2HPO4 |
| Molar mass | 132.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.62 g/cm³ |
| Solubility in water | 572 g/L (20 °C) |
| log P | -2.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa 7.2 |
| Basicity (pKb) | 11.7 |
| Magnetic susceptibility (χ) | −65.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.525 |
| Dipole moment | 6.24 D |
| Chemical formula | (NH4)2HPO4 |
| Molar mass | 132.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.619 g/cm³ |
| Solubility in water | Moderately soluble |
| log P | -2.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 11.7 |
| Magnetic susceptibility (χ) | -57.0e-6 cm³/mol |
| Refractive index (nD) | 1.487 |
| Dipole moment | 6.12 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 109.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1637 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | –283 kcal/mol |
| Std molar entropy (S⦵298) | 96.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1590.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -283 kJ/mol |
| Pharmacology | |
| ATC code | V06DF04 |
| ATC code | V04CX10 |
| Hazards | |
| Main hazards | Not hazardous according to GHS classification. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | No signal word |
| Hazard statements | Hazards not otherwise classified (HNOC) |
| Precautionary statements | Wash hands thoroughly after handling. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Explosive limits: Non-explosive |
| Lethal dose or concentration | LD50 (Oral, Rat): 6,940 mg/kg |
| LD50 (median dose) | 12,690 mg/kg (Rat, oral) |
| NIOSH | WW4250000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Main hazards | Not a hazardous substance or mixture. |
| GHS labelling | GHS07 |
| Pictograms | GHS07,GHS09 |
| Hazard statements | Hazard statements: Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Precautionary statements | Precautionary statements: P264 Wash hands thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P301+P312 IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. P330 Rinse mouth. |
| NFPA 704 (fire diamond) | NFPA 704: 1-0-0 |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 (Oral, Rat): 6500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5,000 mg/kg |
| NIOSH | NL297 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Ammonium dihydrogen phosphate Monopotassium phosphate Disodium phosphate |
| Related compounds |
Monoammonium phosphate Ammonium dihydrogen phosphate Ammonium phosphate Ammonium sulfate Trisodium phosphate Potassium dihydrogen phosphate |

