D-Xylose: A Down-to-Earth Look at a Key Carbohydrate
Historical Development
D-Xylose didn’t just arrive on the scientific scene yesterday. People first noticed this sugar in wood way back in the late 19th century, drawn to it during studies on plant matter. Biochemists saw potential, not just because D-Xylose exists in hemicellulose, which forms a good chunk of agricultural waste, but because its structure separates it from the more common six-carbon sugars such as glucose. Over time, the food and pharmaceutical industries dialed in, noticing unique uses that go beyond the basics of table sugar. Refining extraction methods shifted from relying on crude acid hydrolysis to more controlled enzymatic and chemical approaches. This change improved yields and produced a cleaner product, which in turn opened more doors for research and application.
Product Overview
D-Xylose falls into the group of pentose sugars. In plain terms, it’s a five-carbon simple sugar, naturally found in many fruits, vegetables, and hardwoods. Its value pops up often as a diagnostic tool for the detection of gut absorption abilities, but manufacturers also sell it as a low-calorie sweetener. Taste profile lands somewhere less sweet than sucrose but more sugar-like compared to many sugar alcohols. D-Xylose often comes as a white crystalline powder, soluble in water, making it easy to handle in both lab and industry settings. It carries a handful of product codes and commonly ships with the label "wood sugar," especially in the context of food and beverage formulations.
Physical & Chemical Properties
In its typical form, D-Xylose forms needle-shaped crystals, and it dissolves fast in water. Its melting point hovers around 144–146°C. Chemists recognize D-Xylose with the formula C5H10O5, and its optical rotation measures around +18° to +20°, depending on purity and temperature. Solubility drops off steeply in most organic solvents, reinforcing its classification as a sugar meant for aqueous environments. These properties not only affect how it gets used in food and diagnostics but also become key when handling it in bulk during manufacturing or research setups.
Technical Specifications & Labeling
Strict labeling standards govern the packaging and sale of D-Xylose. Companies selling to the pharmaceutical or food industries must issue certificates confirming purity — many lots hit 99% or better. Labels usually state the specific form (D-configuration), batch number, production date, country of origin, and suggested storage conditions. Specifications often highlight low moisture levels (commonly under 1%), negligible presence of heavy metals, and the absence of harmful byproducts from synthesis, reflecting both regulatory demands and consumer appetite for transparency.
Preparation Method
Most large-scale D-Xylose comes out of agricultural biomass. The usual method begins with acid hydrolysis of xylan-rich hemicellulose — think corncobs, birchwood, or straw — where heat and pressure break down the plant material and release D-Xylose. Chemical engineers separate out residues and neutralize acids before crystallizing the sugar from solution. Some facilities switch to enzymatic hydrolysis to avoid forming byproducts or minimize energy waste, and this method delivers a purer yield at the cost of slower processing. Recovery involves repeated purification steps, including activated carbon treatments and fine filtration, before the final drying stage.
Chemical Reactions & Modifications
D-Xylose opens up a lot of options for chemical modification. Through hydrogenation, for example, it transforms into xylitol, a sugar alcohol widely used as a sweetener in dental-care products and low-calorie foods. Oxidation generates xylonic acid or furfural, both valuable in specialty chemicals or as building blocks for bio-based plastics. Laboratories sometimes use D-Xylose as a starting point for synthesizing rare sugars or isotopic tracers. Its five-carbon backbone proves useful in understanding the reactivity and behavior of sugars beyond the more familiar glucose or fructose.
Synonyms & Product Names
D-Xylose travels by many names: wood sugar, Aldopentose, and Xylopyranose pop up regularly on product lists. Food ingredient suppliers favor “wood sugar” given the plant origin, and technical catalogs often refer to it by CAS number 58-86-6. In standards documents, IUPAC sticks to “(2R,3R,4R)-Pent-1-ene-1,2,3,4,5-pentol”, but in practice, most buyers and technicians simply call it D-Xylose.
Safety & Operational Standards
Anyone in the lab or plant needs to treat D-Xylose like other fine powders, using dust-control measures and keeping all surfaces dry and clean to avoid slips or residues. Safety data shows low toxicity and minimal risk with skin contact or inhalation in the amounts used in food or testing labs, but standards require gloves, eye protection, and dust masks during larger-scale handling. Regulatory bodies worldwide approve D-Xylose as safe for food use, subject to purity and contamination checks. The biggest risk in most environments comes from improper storage, where moisture can cause caking or microbial growth, so clear, sealed packaging makes a big impact on both safety and shelf life.
Application Area
D-Xylose doesn’t land in just one spot. It serves as a substrate in lab tests for digestive absorption — the well-known “D-Xylose absorption test” helps doctors check for celiac disease or other malabsorption cases. Food technologists mix it into low-calorie diets and products intended for diabetics, thanks to its low glycemic response. Over the years, bakers have learned to rely on its flavor and browning properties in bread and cookies, where it boosts Maillard reactions without over-sweetening. Biochemists explore its use as a precursor to valuable bio-based chemicals, considering its renewable origin. Dental product designers tap xylitol, made from D-Xylose, to make chewing gum and toothpaste that won’t feed mouth bacteria.
Research & Development
Interest keeps growing in making D-Xylose from non-food biomass as researchers look for ways to turn agricultural or forestry waste into revenue streams. Process engineers tweak hydrolysis and purification processes, searching for better yields and greener chemistry. Pharmacologists test D-Xylose derivatives as antivirals and antitumor agents, hoping to find new roles for this carbohydrate outside its original food and diagnostic test market. Demand for precision nutrition and specialty sweeteners keeps R&D funding busy, especially as consumers ask where their ingredients come from.
Toxicity Research
Toxicological studies on D-Xylose tell a simple story: high doses may lead to mild gastrointestinal upset, but it doesn’t show mutagenic effects or acute toxicity in animal tests. Human absorption tests, often involving children and vulnerable subjects, support its safety in clinical use. Researchers check for hidden risks, such as contamination by heavy metals or residual acid from manufacturing, but these hazards link back to poor processing, not the base molecule itself. Regulatory reviews continue to clear D-Xylose as food-safe in North America, Europe, and Asia, underlining the confidence in its use.
Future Prospects
D-Xylose looks set to play a bigger role as the world tilts toward renewable chemistry. More biorefineries see value in turning plant byproducts into platform sugars, and D-Xylose sits high on the list because it makes so many other chemicals accessible. As people look harder at personal health and environmental claims linked to ingredients, demand for traceable, naturally sourced sugars rises — and D-Xylose delivers on both counts. Health researchers continue to probe its effects on gut microbiota and potential roles in precision medicine. With technology improving extraction and reducing waste, the next generation of wood sugars could break through limits set by cost and scale, making D-Xylose both familiar in food and transformative in bio-based industries.
What is D-Xylose and what is it used for?
Looking Beyond Table Sugar
D-Xylose stands out from the crowd of sweeteners lining store shelves. It comes from plants—hardwood, corn cobs, and straw—where it forms part of the backbone of hemicellulose, one of the main types of plant fiber. Unlike table sugar, D-Xylose is what's called a pentose, meaning its chemical structure holds five carbon atoms. That small difference sets up a whole new world of uses and reactions in the body.
The Value in Health Foods and Research
Many people want to cut down on calories and blood sugar spikes. D-Xylose offers a way to sweeten food and drinks with far fewer calories than sucrose. Folks with diabetes or those watching their sugar can choose products that use D-Xylose as a sweetener. Since it barely raises blood sugar, it comes in handy for diet management and research into alternatives for people dealing with chronic conditions. In the clinic, doctors have used D-Xylose as part of tests for digestive problems—checking how well the intestines absorb certain sugars. The body absorbs D-Xylose differently than glucose, so it helps pinpoint issues in the small intestine's lining. That practical use helps identify malabsorption disorders, helping patients get the right treatment.
Going Further in Industry
Manufacturers lean on D-Xylose for reasons beyond human health. It plays a key role in making xylitol, a sugar alcohol that turns up in sugar-free gum and dental care products. Xylitol tastes sweet but doesn’t feed the bacteria that lead to tooth decay. D-Xylose supplies the starting material for this popular ingredient, and as consumer demand grows for lower-sugar lifestyles, D-Xylose sits close to the starting line for many related products.
Sustainability and the Bigger Picture
Plant sources for D-Xylose make it renewable, and with the food industry searching for cleaner alternatives, it scores points for both function and planet-friendly credentials. Using farm waste like corn cobs to produce D-Xylose gives new value to what used to be thrown away. Researchers keep studying better ways to extract it, hoping for processes that cost less and pollute less. The more efficiently the industry can make D-Xylose, the less stress falls on food security or the environment.
Points to Watch Out For
There’s still room for caution. Too much D-Xylose—like any sugar—may cause digestive upsets in sensitive people. Some reports highlight issues like gas or bloating after large amounts. That teaches us to use moderation and remember that “sugar-free” doesn’t mean consequence-free. For food makers, getting the sweetness level right and giving clear information helps shoppers choose wisely.
Better Choices Through Science and Transparency
As scientists dig deeper, D-Xylose brings promise as part of a shift to better nutrition and less reliance on cane sugar or high-fructose corn syrup. For anyone with a sweet tooth, it opens new paths. For the planet, it means finding value in plant resources that were once overlooked. For people who want something a little kinder to blood sugar, D-Xylose puts another option on the table. The story of D-Xylose isn’t flashy—but for those looking for lower-sugar living, it means progress that tastes just a little bit sweeter.
Is D-Xylose safe for human consumption?
What Is D-Xylose and Where Does It Show Up?
D-Xylose, a sugar found in wood, fruits, and some vegetables, quietly finds its way into food products and lab tests across the globe. It’s a pentose, meaning it carries five carbon atoms, and the body can source it from things like strawberries, broccoli, and even coconut flour. Plenty of nutrition labels don’t list it directly, but certain low-calorie sweeteners and some dietary fibers rely on it or its derivatives. Often, health professionals use the D-xylose absorption test to check how people digest and absorb sugars. Before anyone starts tossing it into recipes or supplements, it’s worth digging into whether it truly belongs in pantries or medicine cabinets.
Checking the Research: How Safe Is D-Xylose?
Science has plenty to say about D-xylose, but not all of it lands on the same page. Several studies point to its safety in reasonable doses, especially in people without underlying digestive or kidney issues. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) marked D-xylose “not specified,” which means they didn’t find any public health risk at usual intake levels. In the United States, the FDA allows D-xylose as a food additive, and it’s generally recognized as safe (GRAS). Sounds like a green light, but real stories from clinics suggest a few speed bumps.
People with clear digestive tracts usually process moderate amounts of D-xylose just fine. Most adults handle a gram or two every so often without complaint. For people whose bodies can’t absorb sugars properly—think about conditions like short bowel syndrome or celiac—D-xylose might ferment in the gut and lead to bloating or diarrhea. It behaves like sorbitol or xylitol in that way. Anyone with kidney disease should take care; reports show D-xylose can build up and even cause metabolic problems in this group. I’ve talked to a few dietitians who keep this in mind before recommending unusual sugar supplements.
Benefits and Downside for the General Public
D-xylose does not spike blood sugar the way table sugar does, making it interesting for researchers exploring diabetes-friendly products. In animal studies, D-xylose sometimes reduced damage related to high-fat diets by lowering spikes from other sugars. There’s a chance food companies will use it more for calorie control, but not enough well-designed clinical trials exist to call it a magic bullet for weight or blood sugar management.
As promising as ‘natural’ sounds, the story doesn’t stop at the label. High doses—well over a few grams per day—aren't common in a natural diet. Most food sources offer very little per serving. Supplement form changes things, packing a dense load the body may not expect. For children, pregnant women, and anyone with a sensitive gut, too much could mean nausea, gas, or loose stools. In my own experience working at a nutrition clinic, people drawn to “rare sugars” sometimes find their stomachs in knots after experimenting, even with substances called safe on paper.
Practical Advice for Safer Use
Regulators say D-xylose works safely in small doses as a test or rare sweetener, but moderation matters. Before popping any sugar alternative, it helps to talk with a doctor, especially if health problems touch the digestive tract or kidneys. Food creators need to keep transparency in mind, labeling ingredients clearly and warning about total serving amounts. Scientists and health professionals still want bigger, longer clinical trials before giving blanket approval. For now, sticking to amounts found naturally in foods keeps risk low.
How is D-Xylose different from other sugars?
What D-Xylose Actually Is
D-xylose shows up naturally in fruits, wood, and some veggies. Often called “wood sugar,” this five-carbon sugar plays a different role from table sugar, high fructose corn syrup, or honey. Glucose and fructose—the sugars lining supermarket shelves—carry six carbon atoms and build up the body’s quick, sweet energy. D-xylose, on the other hand, brings a pentose backbone that the human body digests in its own way.
How the Body Handles D-Xylose
Eat a doughnut, and blood sugar spikes. Glucose floods in, prompting insulin. D-xylose slips through this process at a much slower rate. The body barely triggers insulin in response. Some researchers use D-xylose tolerance tests to check on intestinal absorption because its unusual pathway tracks gut health instead of blood sugar. People who need to cut down on glucose swings, like diabetics, sometimes see D-xylose as a helpful alternative. That said, the digestive tract absorbs D-xylose only in small amounts—and the rest passes through, making it less likely to turn directly into body fat.
Sourcing Matters: Wood to Table
Harvesting regular sugars starts in the field—cane, beets, or corn. D-xylose doesn’t pour out of these crops. Instead, it lives mostly in corncobs, birchwood, and other woody plants. Manufacturers extract it through hydrolysis: intense heat, acid, or even enzymes crack open plant cell walls, releasing D-xylose. Extracting sugar from wood wastes nothing; it brings value to plant matter that winds up as leftovers in other industries.
The Sweetness Difference
D-xylose’s sweetness runs about half of table sugar’s. It doesn’t overwhelm the taste buds but brings enough flavor to sweeten low-calorie foods. Food technologists mix D-xylose with other sweeteners to reduce calories without losing taste. Its lower sweetness sometimes means a product needs more, driving up production costs compared to common sugars.
Digestive Perks and Drawbacks
D-xylose doesn’t ferment in the gut the way other low-calorie sweeteners do. Too much xylitol gives some people stomach aches or sends them sprinting to the bathroom. D-xylose typically causes less GI upset at moderate doses. It also feeds “good” bacteria, supporting a balanced gut microbiome. People with rare hereditary enzyme deficiencies, like pentosuria, should stay cautious—D-xylose can collect in the body if it doesn’t break down properly.
Where D-Xylose Finds a Place
Creative bakers and food developers prize D-xylose for its browning effects. Glucose caramels up, but D-xylose browns even faster under heat. That speeds up crust development, perfect for low-sugar cookies or breads. It pops up in foods for people watching blood sugar, and in research projects looking for new, smarter sweeteners.
What the Future Holds
The world wants lower-calorie foods that don’t taste like compromise. D-xylose checks a lot of boxes: small impact on blood sugar, fewer digestive problems, and a sustainable production process. But the price tag and taste can keep it from widespread use. Chefs, dietitians, and scientists keep tinkering, hunting for ways that D-xylose can lighten up recipes and help manage health—without losing the pleasure of a little sweetness.
What are the side effects of D-Xylose?
What is D-Xylose?
D-Xylose is a type of sugar that shows up in wood, fruits, and some vegetables. Hospitals sometimes use it in tests that help check if the digestive system absorbs sugars the way it should. People will often see it in health food products, used as a sugar substitute. The food industry likes it for its low-calorie content and the promise of a healthier label. Some bodybuilders and health-conscious folks have tried it because it doesn’t spike blood sugar sharply, unlike standard table sugar.
Why Even Care About Side Effects?
Reading the label on a supplement or a “natural” sugar alternative, it’s easy to imagine it’s problem-free. That’s a big mistake. Plenty of ingredients sold on health store shelves or mixed into packaged foods can interact with the body in ways shoppers don’t expect. I’ve learned over time that just because something looks harmless on paper, it doesn’t always treat everyone kindly. Even if a compound is “natural,” that word alone doesn’t guarantee safety.
Common Side Effects Reported
People who have taken D-Xylose report digestive changes more than anything else. For most healthy adults, it tends to pass through without a fuss. Some people, though, complain of bloating, gas, or a feeling of mild stomach upset after consuming it. I once tried a protein bar featuring D-Xylose, and a couple of hours later, my stomach felt a bit off—not serious, but definitely not something advertised on the label.
Larger doses seem to bring out diarrhea and cramping, especially in those with sensitive guts or conditions like irritable bowel syndrome. Children, older adults, and anyone with kidney or liver issues should take extra care—these groups may process sugars differently, and D-Xylose can put a little more stress on those organs.
More Serious Concerns
Although rare, people with D-Xylose intolerance or certain enzyme deficiencies have faced more intense side effects. Cases turn up in medical literature showing kidney function issues when large amounts enter the system over several days. This isn’t something most people need to worry about after a cookie or two, but those using it as part of a diagnostic procedure or in higher therapeutic doses might have real cause for concern.
Doctors sometimes use the D-Xylose absorption test to check for problems like celiac disease. If someone’s kidneys or liver already struggle, introducing high amounts of this sugar might overwhelm the system. One well-known medical reference, UpToDate, points out that any pre-existing renal compromise raises the risk for complications after D-Xylose dosing.
How to Stay Safer
Reading up on ingredients and staying tuned to your own body’s signals offers the best protection. Anyone feeling queasy, or noticing digestive changes after trying a new supplement or “healthy” sweetener, should tell their healthcare provider. Taking anything outside of a doctor’s guidance, especially for those managing long-term illnesses, feels risky.
The food industry usually puts small, safe amounts into products. Trouble really appears when people take larger doses either through supplements or testing procedures. It helps to follow instructions exactly and ask about any chronic illnesses that could affect processing sugars. For most people, moderate consumption won’t spark issues. For others, it pays to be cautious—particularly if stomach or metabolic problems already exist.
Where can I buy D-Xylose and what is its price?
Everyday Value of D-Xylose
D-Xylose grabs more attention lately, especially from folks in food science, researchers, and people working in specialty baking. The compound brings out sweetness without causing the same blood sugar spikes as regular table sugar. Many diabetic and pre-diabetic individuals are swapping out their regular sweetener for D-Xylose. On top of that, some food producers rely on it to create browning in baked goods through the Maillard reaction. So it’s more than just a weird name in a chemistry textbook.
Places to Buy D-Xylose
These days, getting your hands on D-Xylose isn’t as tough as it once was. Several big online retailers and specialty supply shops keep it in stock. For people who need small amounts, e-commerce giants like Amazon and eBay usually have listings from both international and domestic sellers. Just type in “D-Xylose powder” and see what comes up. Food ingredient stores online like Modernist Pantry, PureBulk, or even shops focusing on molecular gastronomy stock D-Xylose for culinary experiments and food manufacturing.
Researchers, labs, or companies needing higher purity or bulk sizing rely on chemical suppliers. Sigma-Aldrich, Thermo Fisher Scientific, and VWR supply D-Xylose in various grades. They handle orders for universities, and health care research, as well as pharmaceutical or food production. Chemical supply platforms such as Alibaba and Made-in-China.com offer even larger bulk quantities, often sourced directly from factories, but these tend to serve the business crowd rather than the average person.
What Does D-Xylose Cost?
The price of D-Xylose runs the gamut depending on where it is bought and in what size. In my own search for sugar alternatives a couple of years back, I noticed grocery-sized packages bottled for home users—around 100 grams—can pop up for $10 to $20 per pack. These usually suit the home chef or someone looking to test out recipes in small batches.
Bulk supply for commercial or institutional use costs much less by weight. One-kilogram shipments cost between $20 and $80 through food and chemical suppliers, depending on grade and purity. Research-grade products, certified for laboratory use, aim higher on the price chart: $50 to over $150 for a kilo, based on how much documentation or purity testing is needed.
Direct-from-factory options can slide the price much lower when buyers commit to 25 kilos or more. I once helped a start-up bakery order raw D-Xylose from a manufacturer in China. They brought down the cost to under $10 per kilo, but the tradeoff was dealing with customs paperwork and paying hefty shipping fees.
Why Quality and Trust Matter
Not every D-Xylose product is created equally. Some off-brand products skip basic safety or contamination checks, especially those sold through open marketplaces. I always look for third-party lab reports confirming identity and purity. Reputation makes a difference—established chemical outfits have long histories, detailed documentation, and a way to reach customer service if issues crop up. Cheap doesn’t always mean smart, especially when trusts in food or lab results are at stake.
Safety matters. D-Xylose is classed as a food ingredient and recognized as safe by regulatory groups like the FDA. Still, quality control varies from one supplier to the next. Anything bound for direct food use or scientific study needs assurances about contaminants, allergens, and correct handling.
Solutions for Better Sourcing
Education stands out. Buyers need to know what to check—such as certifications and supplier reputation. For those new to sourcing specialty ingredients, it helps to start with sample quantities from respected vendors before diving into large orders. Posting in online forums or networking in scientific societies or chef communities can point folks toward good sources and help avoid low-quality stock. I learned through shared experiences, and those nuggets of advice saved me from buying questionable product.
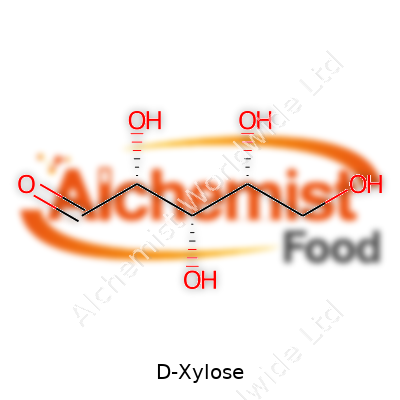
| Names | |
| Preferred IUPAC name | (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal |
| Other names |
Wood sugar Xylo-pentose |
| Pronunciation | /ˈdaɪ.ˌzaɪ.loʊs/ |
| Preferred IUPAC name | (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal |
| Other names |
Wood sugar Xylopyranose Xylose D-Xylopyranose |
| Pronunciation | /ˈdaɪ.ˌzaɪ.loʊs/ |
| Identifiers | |
| CAS Number | 58-86-6 |
| Beilstein Reference | 1720240 |
| ChEBI | CHEBI:28694 |
| ChEMBL | CHEMBL12311 |
| ChemSpider | 7020 |
| DrugBank | DB00155 |
| ECHA InfoCard | 100.038.857 |
| EC Number | EC 200-416-4 |
| Gmelin Reference | 82138 |
| KEGG | C00181 |
| MeSH | D-xylose |
| PubChem CID | 135191 |
| RTECS number | ZE0175000 |
| UNII | F8H60ZQZ2M |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID3022849 |
| CAS Number | 58-86-6 |
| Beilstein Reference | 1720240 |
| ChEBI | CHEBI:28808 |
| ChEMBL | CHEMBL12321 |
| ChemSpider | 504 |
| DrugBank | DB00126 |
| ECHA InfoCard | 100.037.541 |
| EC Number | EC 200-416-4 |
| Gmelin Reference | 3976 |
| KEGG | C00221 |
| MeSH | D-Xylose |
| PubChem CID | 135191 |
| RTECS number | ZH5010000 |
| UNII | Z58X6R1ZXI |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID4044715 |
| Properties | |
| Chemical formula | C5H10O5 |
| Molar mass | 150.13 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.561 g/cm³ |
| Solubility in water | High (glucose-like) |
| log P | -2.46 |
| Acidity (pKa) | 14.15 |
| Basicity (pKb) | 12.38 |
| Refractive index (nD) | 1.605 |
| Viscosity | 4.1 mPa·s (20°C, 50% solution) |
| Dipole moment | 4.51 D |
| Chemical formula | C5H10O5 |
| Molar mass | 150.13 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.52 g/cm³ |
| Solubility in water | 792 g/L (25 °C) |
| log P | -2.2 |
| Acidity (pKa) | pKa = 12.15 |
| Basicity (pKb) | 14.39 |
| Refractive index (nD) | 1.615 |
| Viscosity | Viscosity: 2.2 mPa·s (20% solution, 25 °C) |
| Dipole moment | 2.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -970.55 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2340 kJ/mol |
| Std molar entropy (S⦵298) | 223.0 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -971.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2375 kJ mol-1 |
| Pharmacology | |
| ATC code | A09AX01 |
| ATC code | A09AX01 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Autoignition temperature | 410 °C |
| Explosive limits | Explosive limits: 7–36% |
| Lethal dose or concentration | LD50 Oral Rat 10000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Mouse oral 16500 mg/kg |
| NIOSH | KN6475000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for D-Xylose: Not established |
| REL (Recommended) | 0.08 g/kg bw |
| IDLH (Immediate danger) | Not established |
| Main hazards | May cause respiratory tract, eye, and skin irritation. |
| GHS labelling | GHS07, Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 86°C |
| Autoignition temperature | 410 °C |
| Explosive limits | Explosive limits: 7–19% |
| Lethal dose or concentration | LD50 oral rat 10,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 10,000 mg/kg |
| NIOSH | KTF31250S2 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 0.2 g/kg bw |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Arabinose Lyxose Ribose Xylulose |
| Related compounds |
D-Xylulose L-Xylose Arabinose Ribose Lyxose |

