Copper Sulfate Pentahydrate: The Blue Stone From Past to Progress
Historical Development
Long before modern laboratories made science a career, folks recognized the value of copper salts like copper sulfate pentahydrate. In the days of ancient Greece and Rome, blue crystals showed up in medicine and crafts. Medieval craftsmen steeped textiles in its solution to lock in color, hoping for lasting dyes. In the nineteenth century, fungicide formulas gave new life to agriculture, right as industrialization took hold. Bordeaux mixture, a blend of copper sulfate and lime, saved vineyards from mildew in France and found its way everywhere grapes grew. Laboratories soon began isolating and analyzing this vivid salt, diving into a world of chemical structure and behavior.
Product Overview
Copper sulfate pentahydrate sits on shelves in everything from farm supply stores to science labs under a handful of brand names. These blue crystals, sometimes reaching a striking azure, pack more punch than their beauty suggests. Used for protecting crops, balancing chemistry in pools, supporting chemical experiments, and even feeding animals in small doses, this compound has earned its reputation by proving itself in the real world. Anyone who has worked in farming, pest control, education, or environmental management will recognize its importance.
Physical & Chemical Properties
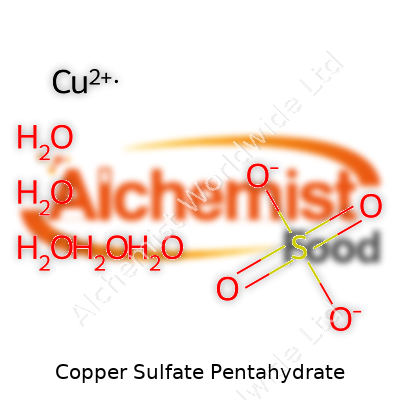
Copper sulfate pentahydrate holds the formula CuSO4·5H2O, giving it those distinctive blue crystals that dissolve quickly in water. On a hot plate, the crystals shed their water and fade to a pale gray-white color, revealing how the water molecules give the salt its deep color. Its molecular weight clocks in at 249.68 g/mol, with five tightly held water molecules setting it apart from its anhydrous cousin. Heat, light, and air influence its stability. For folks mixing chemicals or feeding livestock, knowing its solubility—abundant in cold water and even more so in warm—makes a big difference. These traits open the door to dozens of uses and reactions in sciences and industry alike.
Technical Specifications & Labeling
Looking to buy copper sulfate pentahydrate means inspecting concentration, purity, and the contaminants it may carry along. Frequently, labels spell out the percentage of active copper—often around 25%—plus the presence of heavy metals or arsenic, since nobody wants unwanted extras. Producers stamp their product with batch numbers, date codes, and origin, crucial for tracing back any trouble if a batch breaks bad. Companies must meet tight rules covering substance purity, including ISO-certified processes and regular testing to keep their material trustworthy, especially when it goes in food or water treatment.
Preparation Method
Turning raw copper into copper sulfate pentahydrate doesn't take sorcery, just steady chemical work. Acid attacks on scrap copper or copper ore—usually with sulfuric acid—create a solution full of copper ions. Evaporate this solution, and bright blue crystals grow as water slowly escapes. Large manufacturers use stainless steel vats, run fans and heaters, and keep things clean to keep impurities low. For lab workers, small batches come together with copper wire and beakers, but industrial facilities aim for high output, keeping process controls tight to hit strict standards on every load. This chemical conversion ties together mining, recycling, and manufacturing in a web that keeps agriculture, pools, and research supplied.
Chemical Reactions & Modifications
If you have messed with copper sulfate in school or in chemical processes, you know it's a hungry participant in many chemical reactions. It loves to give up its water with a bit of heat, turning into white anhydrous copper sulfate. Add iron to copper sulfate in solution and you’ll see a red-brown metallic copper grow as iron rusts away, a classic experiment for new chemistry students. Mix it with reducing sugars for Benedict’s test, and it flips colors, helping spot glucose in food or urine. Professional chemists have pushed things further, using copper sulfate as a catalyst in organic chemistry or as a starting point for specialized salts and complexes—always exploring fresh ground.
Synonyms & Product Names
In the real world, a single chemical almost never goes by a single name. Copper sulfate pentahydrate’s alter egos include Blue Vitriol, Blue Stone, and Roman Vitriol. The term “chalcanthite” crops up in geology and mineral collecting circles, even though you’ll see “copper(II) sulfate pentahydrate” on lab bottles. Manufacturers bring their own names to market, some aiming for brand recognition across farm products, pool maintenance chemicals, or science supplies. Origins may shift from one name to another, but the crystalline blue heart stays consistent no matter whose label you’re reading.
Safety & Operational Standards
Handling copper sulfate pentahydrate calls for respect—gloves and goggles at a minimum. On the farm, improper mixing, careless spills, or drifting powder can irritate skin, eyes, and lungs. Drinking water supplies have strict thresholds for copper, so dosing requires care and measured tools. OSHA and EPA set workplace exposure and environmental wastewater limits, with good reason: too much copper builds up and causes harm. Storage protocols demand sealed, labeled drums kept dry and away from acids or food. On clean-up, contaminated soil and waste solutions call for secure disposal—no dumping down drains or sprinkling outdoors. Companies build training programs and audit trails to keep workers safe and the compound where it belongs.
Application Area
Few chemicals cut across industries and daily life like copper sulfate pentahydrate. Farmers and gardeners battle mildew, algae, and root invaders using the compound in liquid sprays and soil soaks. City crews dose drinking water towers or lakes with measured amounts to control algae blooms, with each state setting policies on timing and public notice. Chemistry classrooms turn to copper sulfate as a bright visual for testing, growing crystals, and exploring electrochemistry. Electronics and metalworking technicians use it for plating physical surfaces and etching designs. Animal feeds sometimes receive small copper tonic doses traced back to this compound, boosting trace mineral intake for livestock. It spreads its reach without much fuss, proving handy across generations.
Research & Development
Scientists push beyond traditional uses, exploring new crops, pathogens, and application methods. Researchers track its effects on water ecosystems, seeking lower-dose treatments that still clear algae but leave fish and plants unharmed. Engineers develop slow-release pellets and blends to stretch results and shield workers from dust. Medical teams have posed copper sulfate pentahydrate as a candidate to block certain viruses or bacteria, but toxicity demands careful limits. Every year, peer-reviewed studies show tweaks and offshoots—testing micronutrients in new animal species, copper’s potential role in batteries, or sharper separation techniques for purifying waste streams. A lot of these projects echo the substance’s deep history, showing that this “old” crystal still brings out fresh ideas.
Toxicity Research
Too much copper sulfate pentahydrate blurs the line between medicine and poison. Even small ingestion can trigger nausea and stomach pain, and bigger doses put kidneys and the liver at risk. Pets and livestock pay the price quickest, as their lower body weight leaves them vulnerable to poorly mixed feed or water. Environmental agencies chart copper’s impact on aquatic life, setting upper limits to guard rivers, lakes, and wetland habitats. Researchers have measured the dose-response curve for dozens of animal models, refining our understanding of how and why it harms. Most poisonings stem from accidents or mistaken use, reminding handlers of the need for safety data sheets and regular training.
Future Prospects
Despite its age, copper sulfate pentahydrate isn’t giving up its place any time soon. As governments ban certain synthetic pesticides, natural mineral salts like copper sulfate draw renewed attention for disease control in organic farming. Tech advances point toward intelligent dosing systems, nanostructured delivery particles, and Cu-based catalysts in new battery and hydrogen production research. Environmental programs focus on closing copper cycles, recycling and recovering used copper salts to prevent runoff or waste. As lab tech, agricultural tool, and environmental asset, its value grows each time we discover new angles and solve past issues. The same vivid blue once prized by wheat farmers or dye-makers keeps finding its way into the world’s most pressing projects.
What are the main uses of Copper Sulfate Pentahydrate?
Copper Sulfate in Agriculture
Anyone who’s spent time on a farm knows fungus can wipe out crops before you can blink. Copper sulfate pentahydrate shows up here as a tried-and-true fungicide. Farmers mix it into sprays or blend it with lime to make Bordeaux mixture. This stays on leaves after rains, fighting off powdery mildew, blight, and rust. If grapes or potatoes fail, farmers lose both time and income. Crop diseases aren’t just an inconvenience—they threaten food supplies, especially where resources run thin. Years ago, our family farm relied on Bordeaux mixture to protect tomato plants through wet summers.
Role in Animal Feed and Nutrition
Copper sits among the essential minerals animals need. Not enough and livestock get sick—think anemia in cattle or weak bones in poultry. Copper sulfate works as a supplement. In many regions, adding a dash of copper sulfate to feed keeps sheep’s fleece thick and cattle strong. The danger swings the other way if anyone goes overboard. Too much copper can poison livestock, so dosing isn’t guesswork. In rural communities where animal health means family security, affordable supplements make a difference.
Spotlight on Water Treatment
Once summer heats up, algae blooms spread like wildfire in ponds, reservoirs, and irrigation ditches. If you want to keep fish alive and water clear, copper sulfate is a common go-to. Just a small dose can clear out scummy water, cutting down on odor and ecosystem imbalance. Municipalities and golf courses use it for the same reason. Anyone who’s watched a farm pond turn pea soup green knows clear water is about more than looks—it’s about sustaining life in the pond and keeping irrigation pumps unclogged.
Chemical Industry and Education
Walk into most high school chemistry labs and copper sulfate pentahydrate sits on a shelf somewhere. Its vivid blue crystals grab your attention. Teachers use it for basic experiments, showing how crystals form or how dehydration happens. Chemical factories use it to refine copper, make catalysts, and test for reducing sugars. Even weaving mills use it for certain dyes. As a kid, I marveled at blue copper solutions turning colorless when we tossed in a nail, seeing chemistry leap off the page into real life.
Electroplating and Metalwork
Factories plating metals need copper sulfate—there’s no substitute for reliable copper deposits on wires, pipes, or circuit boards. Electroplating protects against corrosion and builds up surfaces in electronics and decorative work. Reliable copper layers keep electrical connections working and products looking sharp. Many mobile phones and computers hiding in home offices depend on these copper coatings.
Safety: Weighing Use and Impact
Copper sulfate pentahydrate earns points for versatility, but handling it takes care. It can irritate skin and eyes or harm aquatic life if dumped in bulk. Agricultural workers use gloves, and some regions place tight rules on applications near streams or wells. Community education helps avoid accidents. I remember our local extension agent organizing workshops to show safe mixing and disposal practices—an hour invested there prevented costly problems when runoff threatened neighboring wells.
Thinking Toward Solutions
Modern growers turn to integrated pest management, mixing copper sulfate with crop rotation and resistant plant breeds to hold down disease. Fish farm operators pick the lowest effective doses and balance treatment schedules to protect fish and waterfowl. Chemistry educators switch to microscale kits, cutting waste and risks. Ongoing research focuses on slow-release formulas and bio-based controls, seeking choices that protect crops, livestock, and water without tipping nature out of balance.
How should Copper Sulfate Pentahydrate be stored?
Everyday Risks with a Common Chemical
A blue crystal like copper sulfate pentahydrate shows up everywhere from science labs to farms. It looks harmless, almost pretty, but this stuff pulls water from the air and breaks down or clumps over time. Without realizing, leaving it sitting open on a shelf can lead to big headaches and real safety concerns.
Moisture Becomes the Enemy
I remember the first time I used copper sulfate pentahydrate in a lab. I left the container open for just an afternoon and by evening, the once dry crystals looked like mush. This isn’t just annoying; the chemical starts to lose its punch. Moisture turns it into a sticky mess, and if it cakes up or dissolves, you waste material and could even disrupt experiments or treatment in water plants. The chemical also eats away at labels, lids, and shelf finishes, leaving behind corrosion and stains.
Keep it dry or it won’t play nice. A tightly sealed container — not leftovers from lunch, but something rated chemical-resistant — stands between you and ruined supplies. I’ve tried glass jars with rubber gaskets and solid plastic tubs with snap-on lids. Anything less just lets in humidity, especially during the summer months.
Safety on the Shelf: Not Just a Rule, but Common Sense
I see people ignore instructions all the time, but taking shortcuts with copper sulfate pentahydrate brings health risks. The dust irritates lungs, triggers asthma, and burns if it touches your eyes or skin. Copper sulfate can leach into household items if spilled. Children and pets have no business near it, even in a garage or shed.
Store it somewhere dry, cool, and away from food — never on kitchen counters or next to snacks. Lock up the shelf if kids live around the home. Always mark the container clearly. People think, “I’ll remember what’s inside,” but months later, blue powder in a jar looks suspiciously like candy or craft supplies to the untrained eye.
Chemical and Environmental Stewardship
Copper sulfate pentahydrate poses dangers to people, animals, and the environment if it enters water sources by accident. Storing it on the floor, near drains, or in rooms prone to leaks means taking a risk nobody needs. If the container cracks or tips, runoff can pollute the local waterway. Separate this material from anything you eat, drink, or use for food preparation.
Don’t just hold onto it for years thinking it’s fine if the seal looks “good enough.” Over time, even sturdy plastic dries out and fails. Check containers for cracks or broken lids every few months and never mix old crystals with new.
Solutions: Simple but Crucial
I find the best answer stays the simplest: use only chemical-safe, airtight containers, keep them high and dry, mark everything with strong, waterproof labels, and set a calendar reminder to check each storage spot every few months. Don’t cut corners on storage. A few extra minutes saved can cost hours in cleanup and risk, not to mention a fine if you spill chemicals somewhere they shouldn’t go.
Respecting the material protects everyone. Whether for school, work, or gardening, store copper sulfate pentahydrate as carefully as you’d handle medicine in a house full of toddlers. The difference comes down to habits — and those are easy to change before something goes wrong.
Is Copper Sulfate Pentahydrate toxic to humans or animals?
What Copper Sulfate Pentahydrate Does in the Real World
Walk into a farm supply store, the blue crystals of copper sulfate pentahydrate catch the eye. Gardeners rely on it to fight fungus, clean mossy roofs, and rid standing water of unwanted algae. In industry, workers use it in mining, leather processing, and textile dyeing. Swimming pool keepers—even the local aquarium—turn to it for water maintenance. It looks useful, even harmless, sitting in a bag on a shelf.
Toxicity: A Much Bigger Issue Than Most Realize
Behind those blue granules sits a tough truth. Copper is vital in trace amounts for plants, animals, and humans; too much brings real trouble. Copper sulfate pentahydrate can burn skin, eyes, and throat. Swallowing even small amounts sends nausea, vomiting, and stomach pain shortly after. Stories from pets and livestock swallowed a little, then started drooling, staggering, or struggling to breathe. Thousands of cases reach poison control centers every year—and some end in emergency care.
The science leaves no room for doubt. The Environmental Protection Agency says it can damage fish and insects in rivers with concentrations lower than most people guess. The CDC lists symptoms: extreme exposure causes internal bleeding, kidney and liver failure, or death if not treated. Kids sometimes find those bright-blue crystals and mistake them for candy. That mistake leads straight to the ER.
Looking at Real Solutions
At schools and workplaces, safety steps matter more than ever. Gloves and eye protection cost less than a doctor’s visit. Teams who clean up chemical spills get strict instruction: wet wipes for small spills, respirators for larger ones, sealed waste bags for every last scrap. On farms, the shift to biological alternatives helps—fungus-fighting bacteria, plant oils, or even lime solve the root of the problem without risking water supplies or pastures.
For homeowners, safe storage turns out to be the most critical step. Copper sulfate pentahydrate never belongs in the kitchen, bathroom, or near the pet food bin. Storing it in a locked cabinet—out of sight and reach—turns a ten-dollar investment into a life saver. Warning labels help, but nothing replaces common sense. If mixed with water for use, always keep extra containers away from kids and animals. Rinsing containers several times before recycling prevents accidental poisoning down the line.
Why Talking About It Helps Everyone
Family doctors urge parents to program poison control numbers into their phones. Pet owners swap stories at the vet’s office, trying to warn each other about what might seem harmless at first glance. Rural communities with well water keep a closer eye on runoff, knowing a little care today keeps their water safe for years. Plumbers and pool techs who treat pipes or tanks with copper sulfate learn to measure doses with precision, understanding the line between helping and harming.
Taking this chemical for granted has already ended in tragedy for some. By telling the stories, these risks don’t fade into the background. Sharing clear information saves lives, and that’s what matters most.
What is the recommended dosage for agricultural applications?
Understanding Field Realities
Farmers originally taught me something you won’t find in a textbook: a field isn’t a lab. It’s a living system obsessed with unpredictability. Storms appear uninvited, temperatures shift without warning, and every square meter of dirt tells a different story. The recommended dosage for agricultural products isn’t just a number off a label. That number reflects a mix of years in research plots and practical, dirt-under-the-fingernails experience. Applying the right dosage—whether talking about fertilizer, pesticide, or herbicide—has direct consequences on yield, soil health, and pocketbooks.
Why Correct Dosage Matters
Applying less than the recommended amount rarely does the job. A low-dose fertilizer application leaves crops stunted, unable to deliver a good harvest. Under-applying herbicide invites weeds to party in the fields, robbing nutrients and water from the main crop. I spoke with a neighbor who tried to “stretch” his fertilizer bill by going just a bit lighter than suggested. The payoff? Shrunk heads of lettuce—barely marketable—and a patchy stand that needed extra labor to tidy up.
Put down more than guidance, and different trouble appears. Plants can burn, soils may lose their microbial life, and the runoff drags farm economics and local water health into expensive arguments. For example, research from Iowa State University shows nitrogen runoff spikes sharply in fields where recommendations get ignored. It moves from poor returns on fertilizer spending to real risk for nearby rivers.
How Dosage Recommendations Take Shape
The rate stamped on the side of a bag is built from soil tests, plant tissue analysis, and field trials. Researchers tweak weather variables, soil types, and crop varieties, then count beans to see which rates bring the best results at the least cost. These trials try to match what growers use in practice. Labels also factor in local disease pressure, pest populations, and sensitive neighboring crops.
Dosage isn’t magic; it follows facts. For example, the University of California Extension recommends 90–120 pounds of nitrogen per acre for lettuce on coastal soils, depending on the crop’s developmental stage. Go beyond that, and yield just doesn’t climb—the extra money drains away, and local groundwater feels the hit. Labels on herbicide like glyphosate average 22 ounces per acre for many broadleaf weeds, with the caveat you must check which weeds lurk in your field before trusting one number.
Making Sense of the Recommendations
The real world rarely lines up perfectly with test plots. Soil organic matter, past management, irrigation, and plain old weather alter what works. The right dosage often starts with the label—then bends to local context. Farmers walking fields and pulling soil samples spot where rates need small nudges: maybe an extra boost in sandy soil where nutrients leach, or a lighter hand after cover crops boost nitrogen naturally.
Technology keeps moving. Precision agriculture lets growers split a field into dozens of zones, applying just what’s needed, right where it’s needed. Variable rate sprayers don’t just promise better stewardship; they save cash, too. But even with all the tech, nobody replaces the person who knows each field by sight and trusts their shovel.
Practical Solutions for the Dosage Puzzle
Sticking to recommended dosage protects yield, the environment, and the bottom line. Before reaching for the spreader, check up-to-date soil tests and read product labels closely. Tap into your local farm adviser, extension office, or fellow farmers—crowd-sourced wisdom fills in the blanks science leaves behind. Catch runoff and soil erosion early; small fixes, like buffer strips near water, save a field from becoming a fraction of its potential. Take the time to understand why the number exists, not just what the number is. That approach turns a suggestion into a tool that works year after year.
How should accidental spills of Copper Sulfate Pentahydrate be handled?
Why Getting Spill Response Right Matters
Copper sulfate pentahydrate serves a role in farming, chemistry labs, and public works. A simple blue crystal, yet its impact goes far beyond its looks. Spills aren’t just a nuisance; left ignored, copper sulfate can hurt waterways, kill fish, and burn skin. Many farm kids, myself included, learned early that dumping out even a cup of that stuff turns a ditch into a dead zone. For me, watching earthworms shrivel in a puddle was not something easily forgotten.
The Environmental Protection Agency lists copper as a toxin above certain levels. Drinking water shouldn’t carry more than 1.3 parts per million. Fish and aquatic insects struggle at much lower concentrations. If you see blue streaks running toward a grate, realize that copper sulfate will not stop until someone stops it.
Cleaning Copper Sulfate Safely
It’s easy to panic—blue dust on concrete, bright crystals under a sink, maybe liquid running off a lab bench. Take a breath. The most important thing is personal safety. Block off the area: slip on gloves, goggles, long sleeves, and a dust mask if powder is in the air. Even in small labs, accidents can send copper sulfate flying—my hands stung for hours after borrowing someone’s leaky flask in college, and I never ignored splash goggles after that.
Don’t let anyone grab a mop or throw water over it—wetting copper sulfate only spreads the problem. Instead, cover any dry crystals with an absorbent—paper towels work for a teaspoon, clay cat litter or spill pads work for larger messes. Gently scoop up everything with disposable tools—dustpan, scoop, or even pieces of cardboard if that’s all you’ve got. Think like a gardener handling slug bait—slow, steady, collecting as much as possible before anything moves further.
If the chemical falls into soil, dig out the top layer. For concrete or tile, wash the spot only after dry material gets cleared, and use just enough dampness. Catch the runoff with more towels. Place all waste, including gloves, into a sealable plastic bag or bucket.
Disposal and Environmental Responsibility
Many towns don’t want hazardous waste dropped in a landfill, so check city rules before dumping anything. My city hosts a spring clean-up for old paint and chemicals, and the workers ask about everything—better safe than sending copper straight to the river.
Copper in soil can linger for decades. If you spill a big batch outdoors, test the soil later. Some folks use a laboratory, but basic test kits give a ballpark figure. Don’t replant-sensitive crops or dump compost over that patch until you see safe numbers.
Wastewater systems cannot always filter out heavy metals. Instruct everyone on site—family, coworkers, janitors—to call management or spill response immediately if a chemical spill happens. I once watched neighbors quietly hose pesticide into the street, thinking water would solve the problem. Their pond died that same summer.
Prevention as a First Principle
Stores lock up copper sulfate for good reason. Leaky packaging, rusty tools, and open containers create most accidents. Always double-check containers, label everything, and store far from drains or doorways. After encountering too many “borrowed” jars with missing lids or crusty edges, I always took a minute to clean up and put things away right.
Effective training saves fish, soil, and sometimes skin. Anyone handling chemicals needs to know the routine: suit up, contain, collect, notify. It’s about building a culture—a warehouse, a farm, or a classroom—where stopping a spill is common sense, not an afterthought.
| Names | |
| Preferred IUPAC name | Copper(II) sulfate pentahydrate |
| Other names |
Blue vitriol Bluestone Cupric sulfate pentahydrate Copper(II) sulfate pentahydrate Roman vitriol |
| Pronunciation | /ˈkɒpər ˈsʌl.feɪt ˌpɛn.təˈhaɪ.dreɪt/ |
| Preferred IUPAC name | Copper(II) sulfate pentahydrate |
| Other names |
Blue vitriol Blue stone Cupric sulfate pentahydrate Bluestone Copper(II) sulfate pentahydrate Roman vitriol |
| Pronunciation | /ˈkɒpər ˈsʌl.feɪt ˌpɛn.təˈhaɪ.dreɪt/ |
| Identifiers | |
| CAS Number | 7758-99-8 |
| Beilstein Reference | 3589284 |
| ChEBI | CHEBI:31440 |
| ChEMBL | CHEMBL1355 |
| ChemSpider | 86706412 |
| DrugBank | DB09153 |
| ECHA InfoCard | ECHA InfoCard: 029-004-00-0 |
| EC Number | 205-504-7 |
| Gmelin Reference | Gmelin Reference: **87506** |
| KEGG | C14175 |
| MeSH | D003302 |
| PubChem CID | 24462 |
| RTECS number | GL8900000 |
| UNII | VZ8U02L1HF |
| UN number | UN3077 |
| CAS Number | 7758-99-8 |
| Beilstein Reference | 1363775 |
| ChEBI | CHEBI:31440 |
| ChEMBL | CHEMBL1201180 |
| ChemSpider | 23978 |
| DrugBank | DB11200 |
| ECHA InfoCard | 03bcc8d6-7e22-433e-9970-8ea9aa1ef8b7 |
| EC Number | 205-504-7 |
| Gmelin Reference | 97044 |
| KEGG | C01380 |
| MeSH | D003550 |
| PubChem CID | 24851298 |
| RTECS number | GL8900000 |
| UNII | FXG068HH6B |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID2020902 |
| Properties | |
| Chemical formula | CuSO4·5H2O |
| Molar mass | 249.68 g/mol |
| Appearance | Blue crystalline solid |
| Odor | Odorless |
| Density | 2.284 g/cm³ |
| Solubility in water | 22.48 g/100 mL (20 °C) |
| log P | -2.70 |
| Vapor pressure | Vapor pressure: <0.1 hPa (20 °C) |
| Acidity (pKa) | 7.8 |
| Basicity (pKb) | 6.37 |
| Magnetic susceptibility (χ) | '-1.4 × 10⁻⁶ cm³/mol' |
| Refractive index (nD) | 1.503 |
| Dipole moment | 4.45 D |
| Chemical formula | CuSO4·5H2O |
| Molar mass | 249.68 g/mol |
| Appearance | Blue crystalline solid |
| Odor | Odorless |
| Density | 2.284 g/cm³ |
| Solubility in water | 22.3 g/100 mL (20 °C) |
| log P | -4.70 |
| Vapor pressure | Vapor pressure: <0.1 hPa (20 °C) |
| Acidity (pKa) | 7.6 |
| Basicity (pKb) | -6.7 |
| Magnetic susceptibility (χ) | Paramagnetic |
| Refractive index (nD) | 1.503 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 133.4 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -2277.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2276 kJ/mol |
| Std molar entropy (S⦵298) | 333.0 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -2277.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2278 kJ/mol |
| Pharmacology | |
| ATC code | A06AD01 |
| ATC code | A07XA02 |
| Hazards | |
| Main hazards | Toxic if swallowed, harmful if inhaled, causes serious eye irritation, may cause respiratory irritation, harmful to aquatic life with long lasting effects |
| GHS labelling | **"GHS07, GHS09, Warning, H302, H315, H319, H410"** |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H410 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P302+P352, P305+P351+P338, P330, P337+P313, P501 |
| NFPA 704 (fire diamond) | 2-1-1-OX |
| Lethal dose or concentration | LD50 Oral Rat: 482 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 300 mg/kg |
| NIOSH | TC38520 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Copper Sulfate Pentahydrate: "1 mg/m³ (as Copper, dusts and mists) |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | IDLH: 100 mg/m³ |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause damage to organs through prolonged or repeated exposure, very toxic to aquatic life with long lasting effects |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H410 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P302+P352, P305+P351+P338, P501 |
| NFPA 704 (fire diamond) | 2-1-2-OX |
| Lethal dose or concentration | Oral rat LD₅₀: 482 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 300 mg/kg |
| NIOSH | SC8925000 |
| PEL (Permissible) | PEL = 1 mg/m³ |
| REL (Recommended) | 1 - 2 kg/ha |
| IDLH (Immediate danger) | CuSO4: copper compounds, as Cu: 100 mg/m3 |
| Related compounds | |
| Related compounds |
Copper(II) sulfate Copper(II) chloride Copper(II) nitrate Copper(II) acetate Iron(II) sulfate Zinc sulfate |
| Related compounds |
Copper(II) sulfate Copper(II) chloride Copper(II) nitrate Copper(II) acetate Iron(II) sulfate Zinc sulfate |

