Cobalt Sulfate: A Deep Dive into Its Journey and Place in Modern Industry
Historical Development
Cobalt sulfate has roots stretching far back into the early nineteenth century, at a time when curiosity about colored glass and ceramics pushed chemists to isolate vibrant pigments. Before lithium-ion batteries took off, cobalt applications lived mainly in pigment manufacture and metal finishing. My generation associates cobalt sulfate with batteries, but for years, it powered blue dyes on porcelain and played a role in Vitamin B12 research. The post-war chemical boom spurred researchers to ramp up mass production methods, so the modern cobalt sulfate market owes much to decades of steady lab work and demand spikes from growing technology sectors.
Product Overview
This compound often shows up as a reddish-pink salt, and companies ship it out in granular, powder, or crystalline forms. The big players send metric tons out every year to battery plants, electroplaters, and chemical labs. While suppliers highlight purity levels, most buyers care more that the batch dissolves readily and stays free of iron, lead, and trace arsenic. Certification for industrial or electronic applications forces suppliers to stick to a tight spec sheet. Demand shot upward with the rise of consumer electronics, smart grids, and the global push to electrify transport. Cobalt sulfate stocks now link critical minerals, geopolitics, and advanced green tech in a way few expected thirty years ago.
Physical & Chemical Properties
Cobalt sulfate, known for its unmistakable rosy color, melts near 735°C in an anhydrous state. Exposure to humid air pulls in water molecules, shifting its form to hexahydrate, which can get sticky. It dissolves well in water, stays stable at room temperature, and resists oxidation under gentle handling. The sulfate anion contributes to water solubility, which becomes vital in both processing and downstream chemistry, such as making cathode materials for batteries or electroplating solutions. Its density sits just above 2 g/cm³, and the solution’s color intensity gives industry folks a quick purity check before spectrometers come into play.
Technical Specifications & Labeling
Most cobalt sulfate packages reach users with clear, regulated labels. Bulk drums and commercial bags show not just the purity but also trace contaminant levels, batch numbers, and shelf-life. For battery manufacturing, specs often require over 98% purity and trace metals down to low parts-per-million. Producers include labels stating CAS numbers, hazard pictograms, UN shipping codes, and emergency measures in the event of a spill. All this detail reflects strong regulatory oversight in chemical logistics, which became central as supply chains globalized and hazard communication standards rose in lockstep with worker safety campaigns.
Preparation Method
Older chemistry texts talk about reacting cobalt metal or oxide directly with sulfuric acid, which produces the sulfate rapidly. Today, most industrial production uses roasted cobalt ores, like those from copper or nickel mines, leaching the material out and then crystallizing it from acidic solutions. Process engineers dial in the temperature, acidity, and filtration steps so the final product fits strict customer needs. Recovered materials from obsolete batteries and e-waste are gaining traction as secondary sources, helping offset the environmental cost of new mining and steadying cobalt sulfate’s raw material input as market pressures shift.
Chemical Reactions & Modifications
Cobalt sulfate joins a wide variety of laboratory reactions. Mix it with sodium carbonate, and you get cobalt carbonate—a pigment and catalyst. Add ammonium hydroxide to a cobalt sulfate solution, and you pull out cobalt hydroxide, useful for making battery precursors. If you introduce strong reducing agents, the cobalt ion reduces to the metal, which then finds use in superalloys and magnets. Designers of new electrode materials continue to combine cobalt sulfate with other transition metal salts, driving battery innovation through careful modification at the atomic scale.
Synonyms & Product Names
Cobalt(II) sulfate pops up in trade and scientific circles interchangeably as cobaltous sulfate, cobalt sulfate hexahydrate, or even sulfuric acid, cobalt(2+) salt (1:1), hexahydrate. Chemists often refer to the hydrated forms simply as “CoSO4·6H2O.” In supply chain paperwork, it may turn up with European Inventory numbers or, for export purposes, just under “cobalt sulfate powder” or “electrolytic grade cobalt sulfate.” These variations reflect national regulations, regulatory guidelines, and common practice in different regions rather than a difference in chemical identity.
Safety & Operational Standards
Direct handling of cobalt sulfate needs proper personal protective equipment. Inhalation risks stand out, since chronic exposure to dust raises the risk of respiratory issues and can sensitize workers to the metal. Eyes, skin, and mouth need protection to prevent irritation and toxic uptake. Regulatory codes require both site and operator to undergo rigorous training, storage, and ventilation measures. Modern standards call for comprehensive risk assessments, special containment in work areas, emergency showers, chemical-resistant gloves, and proper labeling according to the Globally Harmonized System (GHS). Waste streams get monitored for cobalt ions, ensuring they stay below regulatory thresholds before discharge. Companies facing surging demand have found value in automating parts of the process, which limits hand contact and helps keep accident reports low.
Application Area
Much of the world’s cobalt sulfate heads into battery cathode production, supporting the growing electric vehicle, energy storage, and electronics industries. It plays a vital role in preparations for lithium-ion, nickel-cadmium, and even emerging sodium-ion batteries. Outside batteries, the pigment industry still calls on it for blue and green glass and ceramics. Some goes to agriculture, providing cobalt trace supplements for livestock in soils lacking enough of the element. Electroplating shops value it for imparting corrosion-resistance to steel and for coloring decorative items. New niches pop up every year: from magnetic alloys to catalysts in petrochemical processes, cobalt sulfate serves as a reliable building block.
Research & Development
Lab teams around the globe keep finding fresh use cases and synthesis tweaks. Recent work focuses on recycling technologies and low-waste synthesis to address environmental concerns tied to mining in developing countries. Safer, greener substitution for other materials drives R&D into low-cobalt cathode designs and novel hydrometallurgical processes. Scientists closely track impurity migration, solubility changes, and changes to crystal structure under thermal or electrical stress, as these factors matter for high-performance and next-generation batteries. New forms of cobalt sulfate, such as engineered nanoparticles or hybrid salts, receive intense scrutiny for their potential in medicine or precision electronics.
Toxicity Research
Toxicologists have long mapped the effects of long-term exposure, linking high intake to respiratory, reproductive, and cardiovascular risks. The European Chemicals Agency, EPA, and OSHA list strict occupational exposure limits, tied to studies showing tumor promotion and organ impacts at high doses. Animal research confirms that ingestion builds up in organs; as such, waste management remains a regulatory hot spot for all industrial users. Animal studies and epidemiological data continue to refine these guidelines, with the most advanced research now exploring mechanisms at the cellular and genetic levels. These advances offer industry leaders new ways to reduce exposures and push for safer operating practices wherever cobalt sulfate gets used.
Future Prospects
Ongoing demand for high-performance batteries suggests that cobalt sulfate use will stay central to next-generation energy tech. Global political shifts over critical minerals have led countries to invest in recycling and substitution strategies, yet high-energy density batteries today still rely on well-refined cobalt salts. Anyone looking to the future will see both promise and challenge: labs racing to cut cobalt content and improve sourcing ethics, new environmental regulations, and market shifts as non-cobalt cathodes start coming online. The pathway ahead will likely blend better yield extraction, breakthrough recycling, and deeper transparency through each stage of the supply chain, as the world balances sustainability and technological progress.
What is Cobalt Sulfate used for?
Making Electric Cars Possible
Every time someone plugs in an electric car, cobalt sulfate helps power the future. This pink salt is one of the go-to sources for cobalt, a metal that has turned from an old-school pigment into a linchpin of energy storage. It’s found in almost every lithium-ion battery that sits in a garage or a pocket today. Companies use cobalt sulfate mostly to make battery cathodes—the positive side where energy gets stored and released. The structure of those batteries just would not hold up with substitutes like iron or manganese. The element’s real job: keeping batteries stable, stopping them from overheating, and giving devices a longer life.
Batteries Fueling Demand
Car companies and tech brands hunt for cobalt compounds because of the way the world is heading. Electric vehicles and city buses run on lithium-ion tech packed with cobalt. Phones, laptops, and even backup batteries in hospitals all depend on it. Each car rolling off an electric assembly line can carry 10-20 kilograms of cobalt. Multiply that by millions, and it’s no wonder the world maps cobalt mines as closely as oil fields. In 2023, U.S. Geological Survey numbers said global cobalt demand could triple by the decade’s end, all because people want longer battery lives without the risk of fires.
Health and Nutrition
Many folks overlook another job for cobalt sulfate—making sure animals stay healthy. Cobalt works as a trace mineral in diets, mostly for sheep and cattle. Their stomach microbes use cobalt to make vitamin B12, which is needed to digest food and build muscle. Without enough, livestock falter. Premixes of animal feed and some fertilizers blend in cobalt sulfate to keep everything running smoothly on farms.
Tough Choices: Mining and Ethics
People only talk about cobalt when something goes wrong. Big headlines focus on mining in places like the Congo, where working conditions haunt anyone awake at night. Children sift through dirt, just so a phone or car can last a handful of months longer. Groups like Amnesty International have shared stories and data, showing real risks at the start of the supply chain. As an industry, it's tough to look away when more than half the world’s cobalt comes from a single country, and too much of that from small mines with little oversight.
Building a Better Supply
Better solutions exist. Recycling cobalt from old electronics cuts down new mining. Some firms now pull cobalt out of dead batteries, keeping metals in the economy and out of landfills. Tech companies pledge to source only from audited mines, paying more to make sure hands at the dig site hold real contracts, not just a hope for better pay. Governments and watchdog groups put pressure on brands to follow strict rules and prove their cobalt is conflict-free.
Looking Ahead
I’ve watched as researchers try to dial back cobalt use by swapping in nickel or iron. Still, for now, electric cars, homes, and farms count on cobalt sulfate. Old batteries shouldn’t pile up in closets; cities and companies could trade e-waste for new materials, closing the loop and taking pressure off miners. The transition to cleaner power has to come with cleaner supply chains. The choices made now determine if the future of cobalt comes with hope for workers as well as drivers.
Is Cobalt Sulfate hazardous or toxic?
Cobalt Sulfate: Common Uses and Rising Concerns
Cobalt sulfate pops up in places you might not expect, powering electric car batteries, helping plants grow, and adding color to ceramics. The electric car boom alone has pushed demand for cobalt through the roof. I’ve handled batteries myself and often think about the invisible stuff behind our cleaner transport. The trouble is, cobalt sulfate carries real health risks for people and the environment.
Health Hazards in the Workplace and Beyond
People who work with cobalt sulfate every day find out quickly it’s not harmless. Even small amounts, if inhaled or handled without care, can irritate skin and eyes. Over time, persistent exposure may cause more serious issues, like asthma or a hard cough that won’t let up. The World Health Organization and studies from the Centers for Disease Control and Prevention outline real cases where factory workers have developed lung problems. Some research links prolonged exposure to higher cancer risk.
Ingesting cobalt compounds by accident or through contaminated food can lead to nausea and nerve problems. Cobalt can sneak into the body in a few ways, and none of them are good. Personal protective equipment and proper cleaning make a big difference, but in parts of the world where safety gear isn’t standard, workers are paying the price with their health.
Environmental Impact and Tainted Water
Mining and refining cobalt, especially in places like the Democratic Republic of Congo, leads to polluted water supplies and ruined farmland. Cobalt sulfate from these sites can seep into rivers and reservoirs. Fish and crops end up with cobalt in their tissues, which brings contamination right onto dinner plates. I’ve seen reports out of mining towns where families swim in and drink water high in heavy metals. Chronic exposure for these communities means stunted growth in kids, developmental delays, and chronic illnesses.
Once it gets in the soil, cobalt doesn’t break down quickly. That means farmers may struggle to grow healthy crops season after season. Local ecosystems get thrown off balance, with insects and animals taking the hit.
Balancing Innovation and Protection
The push for greener technologies shouldn’t skip over the mess left behind by their ingredients. Cobalt sulfate supports the growth of renewable energy and powers millions of cars, laptops, and phones, but safety can’t be swept under the rug in the scramble for progress. Responsible sourcing and recycling need real investment. Recycled cobalt not only lowers demand from new mines, it cuts off some pollution at the source.
Better regulations help, too. Companies can trace their raw materials, look for ethical mines, and force suppliers to meet real safety standards. Transparency matters. People deserve to know if their cell phone came at the expense of clean air or sick workers. I trust strict regulations, strong labor protections, and certified supply chains more than vague promises from big industry.
Moving Toward Safer Alternatives
Researchers and tech firms chase after battery designs that skip cobalt altogether. Lithium iron phosphate batteries already power some buses and cars, and scientists work on sodium-ion batteries and other alternatives. These options won’t solve every problem, but each step away from hazardous materials helps. If innovation can make something safer for workers, safer for neighbors, and safer for the planet, I say let’s keep pushing until cobalt sulfate and its risks belong firmly in the past.
What are the storage requirements for Cobalt Sulfate?
Why Worry About Cobalt Sulfate Storage?
Cobalt sulfate pops up in everything from rechargeable batteries to animal feed supplements. The dusty pink powder has a quiet presence behind big changes in energy technology. Yet, it brings a set of practical storage challenges that anyone handling it can’t brush aside. Coming from my time working in a battery research project, I learned that safety and product integrity hinge on the simplicity of properly storing chemicals like cobalt sulfate. Skipping these steps risks contaminating the environment and putting people in danger.
Risks Lurking in the Bag
Cobalt sulfate absorbs moisture from the air. Left out on a humid shelf, it clumps and changes chemically. The product might lose its effectiveness or mess with precise dosing, especially for folks in the animal nutrition field. The real headliner, though, is toxicity. Cobalt compounds can cause respiratory issues and skin problems. In my first lab job, nobody treated “minor” chemicals differently, because all it took was one poorly-sealed jar to trigger a chain of hassles. Good storage starts with the right container. Airtight, properly labeled drums make a huge difference—nothing fancy, just barrels that keep moisture and air out.
Right Place, Right Conditions
Stow cobalt sulfate in a cool, dry, well-ventilated spot. Direct sunlight speeds up breakdown, while basement-level humidity turns powder into sludge. On my shelf, silica gel packets become standard companions in storage bins. Even a simple fan to move stale air helps hold the line against corrosion and odor. One strong lesson: build a habit of reading the manufacturer’s safety sheet, not just scrawling “hazardous” on a label and calling it a day.
Of Rules and Realities
The law puts tight rules around cobalt sulfate in many countries, especially after the rush to mine and process more for electric vehicle batteries. These rules don’t exist just for paperwork’s sake—they came from real accidents. In smaller workshops, folks sometimes ignore “official” guidelines. I’ve seen powder scooped out by hand or left wide open. Even if government inspectors rarely visit, these lapses increase the odds of contamination or exposure. Good companies treat the rules as a best practice, not a box-checking exercise.
Damage Control and Emergency Steps
If powder spills, don’t reach for a broom and hope dust won’t fly everywhere. From my own spill experience, containment works best with gloves, a mask, and a damp cloth to keep particles down. Store all clean-up waste as hazardous material. Nobody gets a second chance with heavy metal exposure—these steps protect people and the nearby water supply.
Toward Better Storage Habits
There’s a push for smarter packaging lately—bags with built-in desiccants and color-changing strips as moisture indicators. Still, all the gadgets in the world mean little if people don’t follow strong habits: label clearly, check seals often, store off the ground. Training staff might sound like bureaucracy, but in reality, it turns out to be the backbone of any safe operation. If workers know what’s in each bin and why it matters, the risk of careless mistakes shrinks fast.
Small Steps, Big Payoff
Cobalt sulfate doesn’t lend itself to shortcuts. It pays to give it a proper place on the shelf and treat it with the respect any toxic substance deserves. Proper containers, mindful handling, good ventilation, and reliable training build a safer workplace and keep supply chains running smoothly, far beyond just following a checklist.
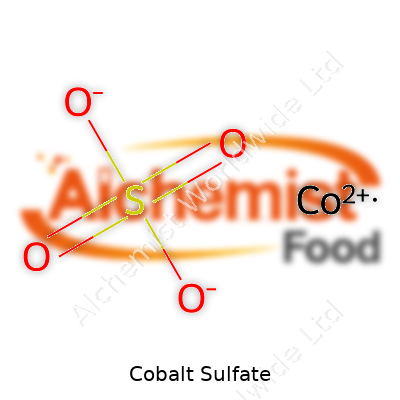
What is the chemical formula of Cobalt Sulfate?
The Straight Answer: CoSO4
Cobalt sulfate’s chemical formula, CoSO4, shows us that it brings together cobalt, sulfur, and oxygen into one compound. Simple as it looks, this formula means different things in different places, from the batteries in electric cars to the fields where fertilizers help crops grow. As someone who appreciates the direct link between the basics of chemistry and real-world impact, seeing those four elements lined up always reminds me of how science drives change outside the lab.
Why Cobalt Sulfate Matters
Cobalt sulfate isn’t just a shelf item in a chemistry classroom. This compound shapes industries. The formula CoSO4 comes up a lot in discussions around lithium-ion batteries, especially since cobalt helps boost battery life and energy density. With electric vehicles getting more popular every year, manufacturers scramble to secure enough cobalt sulfate to keep up. Reports from the United States Geological Survey confirm spikes in demand, each year topping the last.
At the same time, agriculture counts on this material. Cobalt sits in the background of many fertilizers, supporting healthy livestock by helping microbes in the soil produce vitamin B12. Without a steady supply, farms in my community face real challenges. Small numbers on a formula sheet connect straight to how much yield a farmer can expect at harvest.
Roots in Mining and Environment
My own experience growing up near a mining region brings another side of cobalt sulfate’s formula to mind. Extracting cobalt for things like CoSO4 brings environmental and human rights concerns, especially in parts of the world where oversight remains limited. Reports from groups like Amnesty International highlight unsafe working conditions, involving families and children. As a local, I’ve seen what unregulated mining can do—streams run red near the dig areas, community wells turn foul, and no one trusts the fish from the river.
Solutions That Address the Real Problems
One approach starts with clear supply chain oversight. Groups like the Responsible Minerals Initiative urge companies to verify where they get their cobalt. By backing ethical mining operations and investing in transparency, we can reduce the risk of abuses. At the same time, companies ramp up research into battery chemistries that rely less on cobalt, such as lithium-iron-phosphate (LFP) designs.
On the recycling side, efforts continue in regions across Asia, Europe, and North America. Projects focus on recovering cobalt sulfate from used batteries, which saves resources and shrinks waste. I’ve spoken with people who work at these plants, and they talk about pride in giving old materials new life and supporting local economies.
Real-World Impact of CoSO4
This one chemical formula reaches from science labs to rural communities, and all the way to high-tech factories and global trade. Cobalt sulfate (CoSO4) means batteries that run longer, fields that grow better crops, and supply chains that need close watching. Each time I see those letters and numbers, I remember the faces and stories behind the chemistry—and the work we still have to do to keep it fair, safe, and sustainable.
How should Cobalt Sulfate be handled safely?
Understanding the Material
Cobalt sulfate plays a big part in modern technology. Rechargeable batteries, ceramics, pigments—all of these need this chemical somewhere down the line. This usefulness doesn’t make it any less risky to handle. I’ve seen in my own experience how easy it is to underestimate materials that don’t seem immediately threatening. In truth, cobalt sulfate deserves a healthy dose of respect. Toxic dust, skin irritants, and long-term exposures—all are part of the deal. Knowing what you’re dealing with is half the battle.
Why Safe Handling is Non-Negotiable
It’s not hard to get sloppy when you’re in a hurry or short on resources. That’s where people usually run into trouble. Cobalt sulfate can cause rashes, eye problems, or even trigger asthma attacks. Breathing in the dust—especially in a closed space—can do serious harm over time. The United States Department of Labor warns that too much cobalt in the air leads to health problems that don’t show up right away. In factory settings, I’ve noticed how a little vigilance—like cleaning up spills right away—makes a huge difference in people’s health. Many cases of workplace sickness could have been avoided with stronger precautions from the start.
Simple Steps Are Most Effective
Everybody leans on complicated solutions, but the basics work better than most expect. Standard gloves made from nitrile or rubber can block cobalt sulfate from touching the skin. I always tell new workers not to skip goggles or face shields—they handle the threat to eyes a lot better than luck does. In places that process sulfate in bulk, using local exhaust ventilation, such as fume hoods, keeps airborne dust out of breathing range. The right kind of disposable mask—N95 or better—adds another layer of safety without much fuss.
Controlling the Environment
Controlling dust can make or break a safe workplace. Industrial sites that keep floors clean and enforce no-dry sweeping rules tend to see fewer problems. Wet methods, vacuum systems with HEPA filters, and sealed containers all help keep the powder from taking to the air. I’ve seen places turn things around with these small tweaks. In one battery plant, regular inspections uncovered weak spots by storage areas. Fixing them dramatically reduced airborne cobalt readings. Good housekeeping, as simple as it sounds, sets the tone for safety culture.
Training Makes the Difference
Some rules need more than signs on the wall. Everyone in contact with cobalt sulfate should learn about the risks before the first shift. Short, clear training—on how to handle spills, how to check their own protective gear, and how to report hazards—beats over-complicated manuals. Regular training updates help workers remember the stakes. In one workshop, talking out real-life scenarios had more impact than reading a list of safety tips. No one likes to think about worst-case scenarios, but clear practice keeps people sharp.
Looking for Smarter Substitutes
Sometimes the product itself isn’t worth the risk. Industry is always searching for less hazardous substitutes, but until then, strict control stands in for innovation. The two go hand in hand. Research funds for safer alternatives make a difference not just for big companies, but for anyone working in the supply chain.
Moving Forward
Handling cobalt sulfate safely promises better health, fewer accidents, and stronger trust at work. This material probably isn’t going anywhere soon, so the push for consistent routines and investment in new solutions keeps folks safer. That matters both for the workers in the lab coats and the people heading home at the end of their shifts.
| Names | |
| Preferred IUPAC name | Cobalt(2+) sulfate |
| Other names |
Sulfuric acid, cobalt(2+) salt Cobalt(II) sulfate Cobaltous sulfate Sulphuric acid cobalt(2+) salt Cobalt monosulfate Cobalt sulfate |
| Pronunciation | /ˈkoʊ.bɔːlt ˈsʌl.feɪt/ |
| Preferred IUPAC name | cobalt(2+) sulfate |
| Other names |
Sulfuric acid, cobalt(2+) salt Cobalt(II) sulfate Cobaltous sulfate Cobalt monosulfate Cobalt(II) sulphate |
| Pronunciation | /ˈkoʊ.bɒlt ˈsʌl.feɪt/ |
| Identifiers | |
| CAS Number | 10124-43-3 |
| Beilstein Reference | 1691204 |
| ChEBI | CHEBI:30056 |
| ChEMBL | CHEMBL1201561 |
| ChemSpider | 21518 |
| DrugBank | DB11130 |
| ECHA InfoCard | ECHA InfoCard: 100.028.296 |
| EC Number | 233-334-2 |
| Gmelin Reference | 770928 |
| KEGG | C00436 |
| MeSH | D003060 |
| PubChem CID | 24586 |
| RTECS number | GF9590000 |
| UNII | NW9QPS338D |
| UN number | UN3077 |
| CAS Number | 10124-43-3 |
| Beilstein Reference | 1206582 |
| ChEBI | CHEBI:31346 |
| ChEMBL | CHEMBL1201537 |
| ChemSpider | 83601 |
| DrugBank | DB11130 |
| ECHA InfoCard | ECHA InfoCard: 027-004-00-5 |
| EC Number | 233-334-2 |
| Gmelin Reference | 4696 |
| KEGG | C14814 |
| MeSH | D003054 |
| PubChem CID | 24598 |
| RTECS number | GF9170000 |
| UNII | 9P4026P4D5 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | CoSO4 |
| Molar mass | 155.00 g/mol |
| Appearance | Red crystalline granules or powder |
| Odor | Odorless |
| Density | 3.71 g/cm³ |
| Solubility in water | Very soluble |
| log P | -2.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | ~2.0 |
| Basicity (pKb) | -5.34 |
| Magnetic susceptibility (χ) | +3600e-6 (SI) |
| Refractive index (nD) | 1.519 |
| Dipole moment | 0 Debye |
| Chemical formula | CoSO4 |
| Molar mass | 155.00 g/mol |
| Appearance | Red crystalline solid |
| Odor | Odorless |
| Density | 3.71 g/cm³ |
| Solubility in water | 72.5 g/100 mL (20 °C) |
| log P | -2.19 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 1.96 |
| Magnetic susceptibility (χ) | +1600.0e-6 cm³/mol |
| Refractive index (nD) | 1.62 |
| Dipole moment | 3.62 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 96.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -773.0 kJ/mol |
| Std molar entropy (S⦵298) | 96.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -769.9 kJ/mol |
| Pharmacology | |
| ATC code | V03AE08 |
| ATC code | V03AB32 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause allergy or asthma symptoms or breathing difficulties if inhaled, may cause cancer, suspected of damaging fertility or the unborn child, very toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS05,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H302, H317, H319, H332, H334, H341, H350, H360, H410 |
| Precautionary statements | P264, P270, P280, P302+P352, P304+P340, P308+P313, P312, P321, P332+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-3-0-Acid |
| Lethal dose or concentration | LD50 oral rat 424 mg/kg |
| LD50 (median dose) | 4714 mg/kg (rat, oral) |
| NIOSH | BW5150000 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | 15 mg/kg |
| IDLH (Immediate danger) | 20 mg/m3 |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause allergic skin reaction, suspected of causing cancer, may cause respiratory irritation, toxic to aquatic life. |
| GHS labelling | GHS05, GHS07, GHS08 |
| Pictograms | GHS05,GHS07,GHS08 |
| Signal word | DANGER |
| Hazard statements | H302, H317, H319, H334, H341, H350, H360Fd, H410 |
| Precautionary statements | P264, P270, P273, P280, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P314, P332+P313, P337+P313, P362+P364, P391, P501 |
| NFPA 704 (fire diamond) | 2-2-3-Acid |
| Lethal dose or concentration | LD50 oral rat 424 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 768 mg/kg |
| NIOSH | DH2975000 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | 15 mg/kg |
| IDLH (Immediate danger) | *Immediately Dangerous to Life or Health (IDLH) for Cobalt Sulfate is 20 mg/m³* |
| Related compounds | |
| Related compounds |
Cobalt(II) chloride Cobalt(II) nitrate Cobalt(II) carbonate Copper(II) sulfate |
| Related compounds |
Nickel sulfate Copper(II) sulfate Iron(II) sulfate Manganese(II) sulfate Cobalt(II) chloride Cobalt(II) nitrate |

