Cobalt Carbonate: Deep Dive into a Critical Industrial Compound
Historical Development
Cobalt carbonate first drew attention because people needed a reliable way to process cobalt for pigments and later, batteries and catalysts. During the 19th century, mining regions in Europe and later Africa and Canada supplied the feedstock, while chemists experimented with different extraction routes. Early on, cobalt compounds gave glassmakers a strong blue dye, but industries saw broader utility by the early 20th century. As refiners figured out how to handle cobalt ores more safely and efficiently, cobalt carbonate’s profile started to rise. Military demand during the wars for new alloys and specialized materials only pushed research further. Over time, the standards for purity and handling tightened, and cobalt carbonate became a pivot for moving from ore to a whole range of cobalt chemicals.
Product Overview
Cobalt carbonate stands apart for its pink hue and the way it unlocks the chemistry of cobalt. This compound often gets used as a starting material for chemicals, catalysts, and pigments. Powdered cobalt carbonate, easy to spot for its dusty rose color, lands on the desks of ceramics manufacturers, battery makers, and chemical plants around the world. Its use extends beyond pigments—increasingly, the rechargeable battery sector finds new value in the compound’s reactivity, helping charge up countless devices.
Physical & Chemical Properties
Cobalt carbonate shows up as a fine pink powder, insoluble in water but reacts with acid to form soluble cobalt(II) salts—making it handy for chemists shaping further transformations. The chemical formula, CoCO3, points to its role as a metal carbonate. Its molar mass sits at about 118.94 g/mol. Exposure to heat above about 400°C causes decomposition into cobalt oxide and carbon dioxide, a process valuable for preparing high-purity materials. The pink color can shift under certain synthesis or hydration conditions, so the look alone doesn’t always signal purity.
Technical Specifications & Labeling
Manufacturers mark bags and drums of cobalt carbonate with details about purity, lot number, moisture content, and trace metal analysis. Specifications often demand at least 46% cobalt content by mass and scrutinize levels of lead, nickel, and arsenic. Particle size distribution influences how well it works in glazes or in battery precursor mixes. In regions with tough safety standards, labeling rules reflect local hazardous materials codes, such as GHS and REACH registration in Europe. Labels need to call out the dangers of dust inhalation and detail recommended personal protective equipment. Over time, technical sheets have gotten thicker as suppliers face consumer scrutiny and new demands from regulators.
Preparation Method
Cobalt carbonate usually starts from a water-soluble cobalt salt, like cobalt sulfate or chloride. Adding a solution of sodium carbonate or ammonium carbonate kicks off a precipitation reaction, instantly trapping cobalt as the pink carbonate. After filtering and drying, some producers run the powder through calcination steps or advanced milling—especially if they serve the battery industry, which wants highly controlled particle properties. On a broader scale, optimizing energetic efficiency and reducing waste by recycling process water and adjusting reagent use has become a focus, especially as more cobalt gets sourced from recycling streams rather than fresh mining.
Chemical Reactions & Modifications
Drop cobalt carbonate in acid, and it fizzes, releasing carbon dioxide and forming cobalt(II) salts. Thermal decomposition stands out, breaking cobalt carbonate into cobalt oxide at high temperatures, a step central to making cobalt catalysts or precursors for battery cathodes. Adding transition metal ions during precipitation can tweak the structure, leading to mixed metal carbonates crucial for advanced battery chemistry. People in research labs regularly modify this compound to adjust surface area or enhance solubility, sometimes adding organic modifiers to meet demanding industrial applications.
Synonyms & Product Names
Cobalt carbonate pops up under many names, depending on where you look. CoCO3, Spherocobaltite (the mineral form), and cobaltous carbonate all refer to the same base chemistry. For those in ceramics, “cobalt pink” sometimes stands as shorthand. Material safety data sheets list synonyms to keep shipping and regulatory authorities aligned worldwide. Less formal names often spin out of company branding, but the base properties tie back to the same pink powder.
Safety & Operational Standards
Safety teams treat cobalt carbonate as a hazardous dust. Inhaling fine particles over time can cause lung irritation, with some evidence pointing to risks for those exposed on the job for many years. Cobalt itself can trigger allergic skin reactions. Regulatory bodies such as OSHA in the United States and ECHA in Europe set occupational exposure limits and demand thorough SDS documentation. Safe handling expects local exhaust ventilation; gloves and respiratory protection show up as standard PPE in most factories. What’s changed over the last decade is a growing focus on how the dust can affect downstream users and the environment, spurring moves toward better containment, waste management, and employee training.
Application Area
Product managers in ceramics relish cobalt carbonate for intense blue pigments after firing, especially in porcelain and tile. Battery makers, hungry for high-purity cobalt, use it as a key material to make precursors for lithium-ion cathodes—a sector that’s grown fast with the rise of electric vehicles. Chemical companies rely on it as a step in the synthesis of catalysts, vitamins like B12, and magnetic materials. Smaller markets pop up in glass coloring and certain corrosion-resistant alloys. Even as pigment demand stays reliable, energy storage increasingly drives new innovation, and unlocks global competition for consistent supply and improved grades.
Research & Development
Over the years, labs have focused on optimizing yield and minimizing impurities—especially iron and nickel, which hurt performance in batteries. Researchers have refined synthesis to control particle shape and size, often using surfactants and additives to engineer properties at the nanoscale. In academic circles, work has gone into doping cobalt carbonate for catalytic applications or exploring new routes that skip heavy acid use, reducing waste. Sustainability teams dig into recycling spent batteries and industrial scrap to recover cobalt and reuse it as carbonate. Universities and startups both rush to unlock better, safer, and cheaper methods, sometimes using green chemistry and electrochemical processing to lessen the environmental hit.
Toxicity Research
Health professionals point to chronic cobalt exposure as a risk for those inhaling or ingesting soluble cobalt compounds. Animal studies and limited worker exposure reports link cobalt salts to respiratory problems and skin sensitization. The International Agency for Research on Cancer tracked evidence for cobalt metal and compounds, noting some carcinogenic potential when inhaled over a long time. Regulatory guidance tells industry to minimize airborne dust and warn workers about the risks. More effort now goes toward monitoring not just factory air but also downstream sites, as cobalt finds its way into consumer goods. Companies track and report incidents, revising best practices as more data reveal long-term health implications.
Future Prospects
Cobalt carbonate’s direction ties directly to global demand for lithium-ion batteries. As electric vehicles and renewable energy storage scale up, more manufacturers want premium cobalt intermediates with tight control over contaminants. Researchers chase alternatives to cobalt, but so far, few match its blend of energy density and thermal stability. With pressure mounting on supply chains, recycling scrap and spent batteries into new cobalt carbonate streams looks promising. Policy shifts, investment in responsible mining, and new recycling technology could shape availability and costs. As demand grows, those who keep a close eye on safety and environmental impact while delivering high-purity product are poised to win trust from both regulators and end-users.
What is Cobalt Carbonate used for?
Where You Find Cobalt Carbonate
Pink dust like cobalt carbonate might not catch your eye in raw form, but it shapes a surprising number of things. I remember watching my uncle, a lifelong ceramicist, work strange chemicals into his glazes. Cobalt carbonate brought out beautiful blues in his pottery. Artists and factories bank on it for those deep hues in ceramics and glass. That pop of color doesn’t come easy; without it, blue glazes look washed out and dull.
Fuel Cells, Batteries, and Tomorrow’s Tech
Modern life leans on rechargeable batteries. Open up a lithium-ion cell, you’ll find cobalt at its core. More of it comes from cobalt carbonate, a crucial ingredient for making battery-grade cobalt. Electric vehicles, smartphones, laptops—these rely on stable and powerful batteries. Cobalt carbonate gets processed to supply that demand.
High demand pushes prices up and shapes supply chains. Companies search for steady, ethical sources. According to the Cobalt Institute, half the world’s cobalt heads for rechargeable batteries every year. With green energy goals growing, batteries need to last longer while keeping safety high. Pure cobalt carbonate helps deliver better batteries, so it matters a lot behind those headlines promising cleaner cars and tech.
The Bright Side of Chemistry
Cobalt carbonate does more than color or charge things. I spent a summer in a metalworking shop watching locals use it in alloys. They needed metals that wouldn’t rust or overheat. Cobalt helped toughen superalloys for jet engines and turbines. Some coating experts rely on it to make magnets and dyes, too. Without cobalt compounds, tech would slow down, engines would fail sooner, and colors wouldn’t pop on cars and phones.
Risks, Hard Truths, and Better Ways
Cobalt doesn’t always come clean. Mining in some regions, like the Democratic Republic of Congo, sees rough labor conditions and environmental risk. Unethical mining poisons rivers and sickens communities. Knowing this turns a simple pink powder into a global concern. People want to know their electric cars aren’t driving someone else’s suffering.
Transparency and recycling matter. Companies now track their cobalt back to its source. Tech firms including Apple and Panasonic talk about "closed-loop" recycling. They collect old devices and recover cobalt for new batteries. Sticking with recycled cobalt cuts demand for new mining and improves supply security.
What’s Next?
Cobalt carbonate holds real value, but questions of cost, source, and health will stay in sharp focus. The world’s pushing for fairer mining and greener supply chains. Research labs look for battery chemistries that use less cobalt or none at all, but replacing it takes time.
Smoother technology, brighter colors, and green energy dreams hang on this small compound. Plenty of folks never see it, but cobalt carbonate often sits at the start of modern progress—both its ups and its troubles.
What is the chemical formula of Cobalt Carbonate?
Getting the Facts Straight: Cobalt Carbonate’s Chemical Formula
Chemistry keeps life running in ways we might not always notice. Take cobalt carbonate, a deep pink or purple solid used in industries from ceramics to vitamins. Its chemical formula—CoCO3—sounds simple, but there’s real value in knowing why formulas matter outside a classroom. CoCO3 means each unit contains one atom of cobalt, one atom of carbon, and three atoms of oxygen. This ratio is crucial for anyone working with the compound, whether in a research lab, a ceramics workshop, or a factory blending pigments.
Where Cobalt Carbonate Shows Up in Real Life
Cobalt is often called a minor metal, yet it carries a lot of weight in technology and manufacturing. Take glassmakers: they use CoCO3 to produce blue hues that decorate glassware found in homes everywhere. Potters rely on it to glaze ceramics with colors that don’t fade or wash out. Companies that make nutritional supplements sometimes use it as a source of cobalt, since cobalt plays a role in vitamin B12, a nutrient the human body can’t do without.
It turns out, CoCO3 isn’t only about color and nutrition. Battery manufacturers, especially those focused on lithium-ion types, use cobalt-based materials to store and release electricity more efficiently. The rechargeable batteries in smartphones and electric vehicles owe some of their power to cobalt. Of course, battery companies might not use cobalt carbonate directly, but this compound often steps in as a precursor during chemical processing. Missing these connections could mean overlooking how modern conveniences reach our hands and homes.
Why Formulas Hold Practical Value
No room for mistakes in material science. Getting a formula wrong can mean losing money or, worse, creating unsafe compounds. I’ve talked with people in ceramics who learned the hard way: skip precision with something like CoCO3 and the glaze comes out the wrong shade or won’t stick. The chemical formula tells the craftsman, technician, or researcher exactly what they’re working with each time. That reliability supports each link in the production chain—from mining cobalt ore to mixing a pigment batch.
Addressing Issues Around Cobalt
Cobalt’s story isn’t always rosy. Most of it comes from countries where extraction raises both environmental and humanitarian concerns, including child labor and pollution. People in the research and manufacturing worlds face pressure to make supply chains more transparent and responsible. Some companies are shifting toward recycled cobalt or seeking alternative materials, reducing reliance on newly mined product.
On a technical level, improvements in battery design can lower the cobalt needed per unit of energy stored. Some scientists work to develop completely cobalt-free energy solutions, while others establish better tracing of mineral sourcing to cut out problematic suppliers. Even the arts community—ceramicists, glaziers, and glassmakers—are starting to ask questions about the origins and impacts of the minerals they choose for their work.
Building Practical Chemistry Awareness
The chemical formula of cobalt carbonate—CoCO3—is more than a sequence of letters and numbers. Understanding what this formula stands for helps people make real world decisions about sourcing, production quality, and safety. With growing attention on sustainable practices, those who use cobalt carbonate gain an edge by paying attention not just to formulas, but to their impacts upstream and downstream.
Is Cobalt Carbonate hazardous to health?
Looking Beyond Labels
A lot of chemical names sound frightening, and cobalt carbonate often finds itself on that list. The pink powder shows up in ceramics, batteries, animal feed, and some vitamin supplements. It’s not a household word, but for folks working in labs, manufacturing, or industries using pigments and alloys, it’s familiar—and not always in a good way.
The Risks Up Close
Cobalt isn’t made for unchecked handling. Breathing in its dust, for example, can irritate airways pretty fast. According to the Centers for Disease Control and Prevention, regular exposure over months or years can set off asthma-like symptoms, chronic cough, and, in some situations, scarring in the lungs. The skin doesn’t get away clean either. Touching the powder without good gloves sometimes brings on rashes or allergic reactions. Years ago, folks in ceramic studios found this out the hard way—red knuckles, dry skin, and after a while, hospital visits.
Eating or drinking cobalt carbonate, on the other hand, causes a different set of problems. If swallowed in larger quantities, the body might deal with nausea, vomiting, and, over time, even heart trouble. Too much cobalt can mess with thyroid function and affect nerve health as well. A 2012 review published in the Journal of Occupational Medicine and Toxicology laid out those risks in plain numbers. It’s true that the body needs tiny traces of cobalt, but an overload brings on classic symptoms of heavy metal poisoning.
Why Worry About This Compound?
Folks sometimes underestimate powders like this, maybe because they look harmless or fit into everyday products. In reality, the trouble often comes from the hidden exposures. Workplaces where employees grind, blend, or mix compounds kick up airborne dust unless good ventilation is in place. Many might think a cheap mask or a cracked window gets the job done, but studies show higher risks in places ignoring proper respiratory protection. No one walks through a cloud of powder and forgets it.
In the past decade, more countries have started tightening regulations about storing and handling cobalt compounds. The Occupational Safety and Health Administration (OSHA) sets maximum exposure limits at 0.1 mg/m3 over an eight-hour work shift for cobalt dusts and compounds. Still, accidents and violations happen, usually when training slips or safety gear isn’t kept up to date.
The Role of Companies and Workers
Companies owe it to workers to foster a solid safety culture. Clear rules, regular medical check-ups, and up-to-date personal protective equipment matter more than a few warning posters on the wall. From what I’ve seen in small ceramics shops and big factories, the difference between safety and danger shows up not only in training sessions but in everyday routines—how folks handle spills, track exposure times, and check air filters.
Workers, too, have to step up—asking questions, reporting poorly maintained gear, and looking out for symptoms early. My experience with longtime technicians taught me that early reporting of coughs or strange skin problems gets results. Companies usually respond when staff speak up together.
Paths Toward Better Safety
Safer substitutes, stronger engineering controls, and transparency in the supply chain help cut down the hazard. Some plants now run with nearly enclosed systems, keeping dust where it belongs. Advocacy groups push for stronger limits worldwide. Sharing information and challenging old rules will likely make exposure even rarer. At the end of the day, no powder or compound—pink or otherwise—should ever come before somebody’s health.
How should Cobalt Carbonate be stored?
Real Concerns Behind the Storage
Cobalt carbonate sounds obscure until you realize it plays a role in batteries, ceramics, and pigments. For anyone working with this powder, mishandling raises more than regulatory headaches — it brings real health and safety concerns. Breathing in fine cobalt dust or letting it contaminate the environment isn't just bad form; it can harm workers and the local community. Some facts stick: repeated exposure to cobalt compounds has been linked with lung and heart issues, while environmental leaks can affect local water quality. So the way this material gets stored deserves straight talk.
Steps That Make Storage Safer
Taking responsibility for cobalt carbonate starts at the receiving dock. Keeping the material in tightly closed containers keeps moisture and air out. I’ve seen smart shops invest in strong, lined steel drums with gasketed lids. These containers do more than prevent spills; they form a real barrier against air and accidental bumps. Choose a spot away from busy foot traffic to park these drums. If a container gets knocked over and splits open, nobody wants to sweep up pink dust scattered across a crowded shop floor.
Store cobalt carbonate in a cool, dry place. Humidity invites unwanted chemical changes. Dampness can clump up the powder and change how it behaves in production processes. Temperature swings — especially in areas with poor ventilation — can make some compounds break down or even off-gas. A climate-controlled storeroom offers peace of mind.
Why Labels Aren’t a Mere Formality
Skimping on clear labeling is a rookie error. I remember a manufacturer who tossed leftover cobalt carbonate in a used container marked only with a Sharpie that soon rubbed off. A worker treated it as an inert material, leading to unnecessary exposure. Every container should list not just the chemical name, but also hazard warnings, date received, and who signed off. Labels should withstand spills and wipe-downs — standard paper tags just won’t cut it. OSHA outlines clear labeling requirements; following these reduces guesswork and mistakes.
Addressing Spills and Handling Leftovers
Leaks and spills are inevitable, but planning for the mess makes a difference. Store absorbent materials and personal protective equipment near the site. I once saw a crew use just a cheap dust mask and a broom during a cleanup, which barely did anything for airborne dust. Real spill kits with gloves, N95 masks, and disposable coveralls keep workers much safer. All waste, including cleanup scraps, belongs in dedicated hazardous waste bins — not the regular dumpster out back.
Training Matters More Than Gadgets
Fancy storage systems only help if people actually know the risks. Basic training goes further than posters on the wall. People need to know why cobalt carbonate is dangerous and learn hands-on cleanup and emergency response. Clear communication — not jargon — builds respect for the stuff.
Final Thoughts on Practical Storage
Safe storage of cobalt carbonate comes down to caring about the people handling it and the community around them. Using the right containers, keeping the storeroom dry and cool, labeling everything clearly, tackling spills fast, and investing in real training all pay off. These steps aren’t abstract compliance — they protect real people, prevent costly accidents, and show respect for the rules. Facing these details head-on makes everyone feel a little safer at work.
What is the appearance and color of Cobalt Carbonate?
Getting to Know Cobalt Carbonate
Anyone who has spent some time in a chemistry lab might recognize cobalt carbonate from its distinct color. The moment you open the container, a dusty, reddish-pink powder stands out. This isn’t your everyday brown or white laboratory chemical. That pink tint comes straight from the cobalt metal sitting in its divalent, or +2, oxidation state. Dull? Never. Even folks working outside the lab—like those in ceramics or pigments—often note how that color brings a certain energy to their materials.
What Sets That Pink Apart
Cobalt carbonate doesn’t just look pink for no reason. This unique shade appears because of the way cobalt bonds with carbonate ions. Instead of producing a dull tone, the chemical structure grabs visible light in a specific way and reflects back that rose or even mauve look. In my own high-school chemistry classes, students always crowded around when we handled cobalt compounds, just because nothing else looked quite like them.
Crystals of cobalt carbonate do show up sometimes, though that’s less common than the basic powder form. Those crystals still carry that deep pink-to-violet color, only their surface catches the light slightly differently, sometimes making the compound sparkle. If the powder is especially fine, it seems even more vivid, almost as if it could rub off onto your fingers. Safety tip: always use gloves, not simply for the staining but because cobalt compounds require careful handling.
Why Appearance Matters Beyond the Laboratory
People in industry care about cobalt carbonate’s color, not for style points but for practical reasons. Pigment makers seek that rich pink color for ceramics and glass glazes, counting on the compound to deliver a steady, repeatable result. If someone swapped in a faded or off-shade batch, the final product wouldn’t turn out the way it’s supposed to.
Science backs up the importance of that color consistency. According to studies from materials science labs, even small impurities—like trace metals—change how cobalt carbonate looks as well as how it functions. Quality control samples from pigment factories often get checked under high-powered microscopes for just this reason.
Handling Safety and Environmental Risks
Pink or not, cobalt carbonate isn’t something to treat lightly. My time working alongside research chemists taught me that even beautiful compounds pack some risks. Powders like cobalt carbonate can become airborne easily, so inhaling them means you’re exposing your body to a toxic heavy metal. It pays to remember that safety glasses, a mask, and gloves aren’t overkill in these situations.
The mining and refining of cobalt also raise ethical and environmental concerns. A lot of cobalt comes from regions with poor environmental controls and tough working conditions, putting pressure on manufacturers to trace sources and stick to responsible suppliers. Greater transparency in the supply chain is one step in the right direction.
Finding Solutions and Responsibility
Industry doesn’t stand still—even when it comes to a compound as old as cobalt carbonate. Manufacturers continue working on safer formulations and digital labeling to trace every step in the production chain. Labs now experiment with ways to recycle scrap material and reduce hazardous waste. Whenever I talk to younger chemists, I remind them that good science isn’t only about making discoveries but also about considering the legacy our choices leave in the world.
What is Cobalt Carbonate used for?
The Backbone Behind Vibrant Colors and Batteries
Cobalt carbonate doesn't stand out in day-to-day life. Most people don't see it or handle it, yet it shapes a lot of products and technologies many use, sometimes without realizing it. I've spent years covering materials science and tracing raw ingredients from mine to manufacture. Cobalt carbonate ranks among the lesser-known chemicals, pressed into service far from the spotlight.
In art and construction, this dusty pink powder finds a home in ceramics and glass. If you admire a deep blue vase or a striking architectural tile, there's a good chance cobalt carbonate played a part. Artists and craftsmen mix it into glazes to develop those distinct, durable blues that resist fading. This use goes as far back as ancient Egypt and China, showing how long humans have relied on this mineral for color.
Catalysts and Chemistry
Industries turn to cobalt carbonate for more than color. The chemical stands out as a feedstock—a starter material—for making other cobalt compounds. Cobalt catalysts help refineries break down crude oil and remove sulfur. Automobile exhaust systems rely on refined cobalt products, the roots of which often begin with this old powder. Clean fuel owes its progress to the refinement that cobalt carbonate enables.
I've spoken with chemists who prize its role in pigments and catalysts. Without the steady supply and correct grade of cobalt carbonate, plants stall and colors in finished goods shift for the worse. So behind the scenes, material consistency and traceability carry real influence. Errors here ripple through the supply chain, hitting quality and cost.
Batteries and the Race Toward Electrification
Jump to the biggest headlines—lithium-ion batteries. The rise of electric vehicles and devices puts cobalt on center stage. Many battery makers use cobalt as a stabilizer, helping batteries last longer and charge more safely. Some of that cobalt gets sourced from compounds derived from cobalt carbonate. The world now pays close attention to where this mineral comes from, since demand has soared.
I've tracked reports from miners in the Democratic Republic of the Congo—a major supplier of cobalt. Their stories show what the rest of us rarely see: Cobalt mining links directly to supply chain ethics and environmental impact. Many tech companies and battery makers feel pressure to provide transparency about where they get their cobalt and how workers are treated. Poor management at the source affects product reputations and consumer trust down the line.
Challenges and Responsible Sourcing
Health and safety matter from mine to factory. Cobalt carbonate dust, under certain conditions, can harm those who handle it without proper gear. Companies respond with strict safety plans and air quality monitoring, but small-scale miners often work without these precautions. Regulatory bodies set exposure limits to protect workers, yet patchy enforcement sometimes leaves gaps.
Researchers are now exploring new battery designs to limit or eliminate the need for cobalt. This hunt for alternatives shows how dependent modern technology still is on a handful of key materials. Recycling programs grow in importance, pulling cobalt out of old batteries and devices and feeding it back into the manufacturing loop.
Looking Ahead
Decisions about sourcing, protecting workers, and managing health concerns shape where cobalt carbonate fits into modern industry. Getting these elements right secures product quality, keeps people safe, and steers innovation toward a cleaner future. There’s no substitute for clear tracking, ethical practices, and sustained investment in finding new ways to use—or replace—cobalt in the products we all rely on.
Is Cobalt Carbonate hazardous to health?
What Cobalt Carbonate Really Means in the Workplace
Cobalt carbonate shows up most often in batteries, pigments, and as a supplement for animals. Out on the plant floor, in battery factories or ceramics workshops, it’s a pinkish powder that ends up on work benches, aprons, employee hands. Ask anyone who’s handled bag after bag — the dust gets everywhere if you don’t pay attention during measuring or mixing.
Heath Risks People Face
Cobalt itself isn’t something our bodies need a lot of, but the body still recognizes it. Breathing in too much cobalt carbonate powder brings tightness in the chest, shortness of breath, and the coughing doesn’t quit after a sip of water. That’s the acute exposure. Over months or years, the number of reports about wheezing, asthma, and weakened lungs grows higher — especially for workers grinding or pouring powders every week without masks or showers on-site. Some studies connect high, ongoing exposure to heart problems, especially in crowded workshops with weak ventilation.
Sparks fly in recycling plants as lithium-ion batteries break open. Research from the National Institute for Occupational Safety and Health shows that small cobalt particles in the air easily reach deep in the lungs. Repeated exposure — even at lower levels — slowly adds stress to the body’s organs, as cobalt builds up in tissues. People sometimes get skin rashes from touching the powder, and persistent irritation can lead to open sores or eczema.
Health authorities, such as the International Agency for Research on Cancer, have flagged cobalt compounds as possibly carcinogenic. This doesn’t mean every worker develops cancer, but compared with the average job, handling these powders carries more risk unless the environment controls exposure.
Personal Experiences with Safety Measures
I’ve worked in a ceramics supply warehouse where cobalt carbonate storage was right next to other metal oxides. The first months burned through three pairs of gloves, and blue tape on the doors read, “Respirators Required.” But enforcement depended on who walked by. Sometimes, after a busy shift, hands came home red-raw and itchy. The safety officer used to insist on vacuuming with HEPA filters and restricted dry sweeping, which kicked up so much loose dust that by lunch, lunchroom tables needed a full wipe.
Small breaks, like proper wipe-downs and keeping work clothes separate from regular clothes, cut down accidental exposure. Still, without regular safety training or good ventilation, it’s easy to get sloppy. I saw a crew member stop using gloves, only to develop severe dermatitis after three weeks.
Solutions for Lowering Harm
Training matters more than most companies realize. Proper respirator fitting, scheduled glove changes, and stricter cleaning routines make the biggest difference. Ventilation upgrades turn out far cheaper than paying for lost workdays or medical bills, especially in small companies. I’ve watched a shop move their powder-mixing area to a room with negative pressure and every worker’s coughing stopped after two months.
Labeling and handling protocols matter. Routine skin checks, access to showers, and proper laundry services bring down long-term effects and give employees a sense of security. Not everyone has access to expensive engineering controls, but even basic steps like full face shields, disposable aprons, and restricting food in the workspace dramatically reduce risk. Tighter rules from regulators have pushed many industries to clean up after years of neglect, and it pays off — both in worker health and in reduced downtime during inspections.
Bottom Line for Everyday People
Cobalt carbonate isn’t something to fear if it’s respected. Outside of shops and factories, risk to the public runs low, but for anyone in the thick of production, habits matter. It’s a case where following rules and demanding the right tools means fewer sick days, fewer regrets, and a healthier workforce. Safety isn’t about red tape — it’s about having the energy and lungs to walk out at the end of every shift.
What is the chemical formula of Cobalt Carbonate?
What Makes Cobalt Carbonate Unique?
In chemistry classes, folks get used to jotting down chemical formulas and moving right along to the next compound on the list. Cobalt carbonate doesn’t often get a spotlight, but its formula—CoCO3—has more packed into it than some might guess. The combination of cobalt (Co) and carbonate (CO3) puts this compound at the intersection of color chemistry, battery tech, and even agriculture.
Getting Familiar With CoCO3
Cobalt itself shines in industry for its magnetic properties and ability to impart deep colors. Its salts often pop up bright pinks or reds, and cobalt carbonate is a solid starting point for several uses. Mixing cobalt and carbonate in this form gives CoCO3: one cobalt atom pairs with one carbonate ion. Under regular conditions, it shows up as a dull pink powder.
Why Should People Care About This Compound?
CoCO3 has roots in several fields. Ceramics folks lean on it to get blue glazes in pottery, a tradition stretching back generations. Artisans know the shade cobalt delivers—no digital substitute comes close. And in the ever-expanding world of rechargeable batteries, cobalt compounds (including ones derived from CoCO3) make up cathodes in lithium-ion cells. Your phone and electric car rely on the chemistry that begins with this pink, unassuming powder.
Farmers caught up with it, too. As a micronutrient, small doses of cobalt carbonate blend into livestock feed. Cobalt keeps ruminants healthy and helps prevent costly vitamin deficiencies, a lesson folks learned the hard way in the early twentieth century. A missing ingredient like cobalt can upend an entire operation. That matters in regions where every blade of grass and every gram of supplement makes a difference.
Challenges—And Solutions That Deserve Attention
Mining for cobalt grabs headlines because of supply chains. Congo supplies most of the world’s cobalt, and mining there often sparks worries: child labor, pollution, and unsafe work conditions. These aren’t just distant headlines—they affect families and communities that depend on the land. Real change starts with digging deeper into sourcing. Companies can demand more transparency, third-party audits, and fair pay for miners.
Cobalt carbonate’s formula doesn't change, but how it’s sourced and used can shift dramatically. Developing recycling programs for batteries helps, since discarded electronics still hold valuable metals. Researchers keep chasing ways to recover cobalt without harming the environment. Support for closed-loop systems will ensure people and planet both come out ahead.
Building Trust With Facts
CoCO3 is a simple formula on paper but links to bigger issues. Experts from materials science to global supply chain management echo the point: understanding chemical sourcing is a shared responsibility. According to the United States Geological Survey, over 70% of cobalt comes out of the Democratic Republic of the Congo, where monitoring transparency has lagged behind demand. The International Energy Agency notes that recycling batteries can ease supply bottlenecks if industry commits to responsible recovery programs.
Everyday Applications—Hidden in Plain Sight
A lot of folks go about their day without knowing the chemistry built into their routines. Pottery glazes, animal nutrition, rechargeable batteries, and paint pigments all tie back to cobalt carbonate’s unremarkable formula. The challenge is using that knowledge to push for greater accountability, better recycling, and thoughtful innovation. Sometimes, looking closely at a simple formula like CoCO3 opens a window to bigger conversations worth having.
How should Cobalt Carbonate be stored?
Understanding the Risks
Cobalt carbonate isn’t just another industrial chemical sitting on a shelf. This fine, pink powder comes with a set of challenges. Inhalation and ingestion both raise health concerns, and repeated, careless handling puts folks around it at risk. Over the years, I’ve seen small lapses — dust escaping from an unsealed bag or a mislabeled drum — create problems that take much longer to fix than most people imagine. Simple practices make all the difference.
Container Choice Matters
A few years ago, I watched a team switching cobalt carbonate from punctured paper sacks into thick-walled polyethylene drums on a sticky summer morning. Heat and humidity were relentless, and the powder clumped up fast. Moisture is not kind to this substance. Exposure to dampness leads to lumps, making the chemical hard to measure and mix, sometimes even reducing its effectiveness for downstream users. So, using strong, sealable containers—think high-density polymer barrels fitted with secure lids—becomes essential. Metal buckets with airtight seals can also stand up to the job, as long as there’s a barrier liner inside to prevent reaction with the metal itself.
Labeling Prevents Headaches
In my own early days on a factory floor, faded or generic labels led to confusion. Teams wasted hours trying to figure out what was inside those grimy 25-kilo containers. Errors slow everyone down and can trigger serious safety incidents. Clear, weather-resistant labels save time and worry. Every container holding cobalt carbonate should show its name, any related hazards, the date filled, and emergency instructions in big print.
Location, Location, Location
The old warehouse trick—shoving hazardous chemicals in the nearest empty spot—never does anyone favors. Storage rooms need good ventilation so that drifting dust doesn’t linger. I remember a nightshift worker coughing after a poorly-sealed bag gave way on a shelf near a vent. With cobalt carbonate, dust should never travel. Shelving with raised edges and spill trays stops accidents from spiraling out of control. Stowing containers on lower racks helps reduce spill risk from dropped packages.
Temperature and Light Controls
People sometimes forget just how much temperature swings can wreck a product. High heat or direct sun speeds up degradation, especially if you store containers near vibrating machinery or steam pipes. Room temperature away from sunlight and sources of vibration gives the best shot at keeping the powder stable for longer stretches. Overhead bulbs should provide enough light for easy label reading but not so much that they heat up the space.
Layered Protection: Gear and Training
Complacency creeps in so quickly. I’ve seen colleagues run out to “just grab a scoop” without masks or gloves, only to spend days battling a rash or chest irritation. Training schedules feel like a hassle in the moment, but no shortcut beats simple personal protective gear: gloves, well-fitting respirators, and full eyewear. Long sleeves and aprons keep powder from getting on skin or clothes. Emergency spill kits should sit nearby, stocked and ready.
Quick Steps When Trouble Hits
Preparation always wins over panic. If a spill occurs, sweeping powder dry and double-bagging waste helps limit spread. Never reach for water unless the safety sheet confirms it’s safe, because adding water can sometimes worsen the situation or make the product harder to clean up. Having emergency numbers visible in storage rooms helps every shift stay ready.
Paying Attention Pays Off
Safe, well-organized storage for cobalt carbonate isn’t just about ticking boxes. People’s health, company reputation, and the reliability of the supply chain all depend on these real-world habits. With every batch received and drum stacked, small steps—right containers, careful placement, and routine checks—add up to a stronger safety culture.
What is the purity of available Cobalt Carbonate?
Cobalt Carbonate on the Market
Purchasing cobalt carbonate for industrial work isn’t like grabbing sugar off a grocery store shelf. You’ll find the stuff in bags or drums stamped with a purity value—typically somewhere between 98% and 99.9%. That tiny percentage gap actually matters. The difference shapes everything from battery manufacturing to pigments in ceramics. I learned pretty quickly the hard way that even a trace of iron, copper, or nickel hiding in those last decimals can cause headaches down the line, especially in tech that absolutely can't handle surprises.
Why Purity Makes People Care
Take battery production as an example. Big companies making rechargeable batteries watch every percentage point with eagle eyes. Too much contamination in the Cobalt Carbonate? You’ll see drops in battery life or performance. I once heard an engineer complain for an hour straight about a half-percentage point dip in metal purity—and he wasn’t exaggerating about the hassle it caused. High-purity cobalt carbonate lets battery producers trust the chemistry, and that spills into longer gadget lifespans and safety on the road for electric cars.
Numbers That Matter
Industry standards keep things in check. Battery-grade material usually falls above 99.5% purity. Mid-grade—think pigment applications for plastics or ceramics—often lands in the 98–99% range. Lower grades do exist, but you’ll rarely find them in advanced tech or top-shelf paints.
That 99.9% label gets a lot of attention. This high-purity grade typically costs more, but buyers snap it up for projects where reliability truly matters. Producers publish detailed specs, laying out what trace metals are present and in what quantities. Transparent sellers let you review the analysis certificates, which shows confidence in their process. Anyone shy about sharing those numbers usually isn’t worth a second glance.
What Gets in and How to Deal With It
Not all impurities come from sloppy work. Cobalt is pulled from ores alongside copper and nickel, so traces of these neighbors sneak in. Modern refining can’t always wipe out every last atom of extra metal unless you throw a lot of money at the problem. Labs test for lead, iron, manganese, zinc—plus water content, which shouldn’t be overlooked. Some buyers run their own independent checks just to sleep better at night. If you’ve ever had a batch that didn’t measure up, the supplier will usually blame their starting ore or outdated processes, but rare plants make that an excuse.
Solutions Worth Talking About
There’s no magic wand for high purity. Responsible sourcing, tighter quality controls, and pressure from buyers for certifications drive improvement. Firms investing in closed-loop refining or better mineral selection tend to get the cleanest product. International standards need to be more closely enforced; it keeps everyone honest. In my own work, building strong relationships with a handful of good suppliers made life much easier. They knew if a sample flunked our tests, we’d walk. Mutual accountability pushes everyone to hit higher standards.
The Big Picture
People working with cobalt carbonate can’t treat purity as a side note. Every percentage point means something down the line—to batteries, colors, and product safety. The companies that set new benchmarks for purity are the ones I trust most, because they recognize that quality builds long-term reputations, not just sales for the quarter.
| Names | |
| Preferred IUPAC name | Cobalt(2+) carbonate |
| Other names |
Sphero cobalt Cobaltous carbonate Cobalt(II) carbonate Cobalt mono carbonate Carbonic acid cobalt(2+) salt |
| Pronunciation | /ˈkoʊ.bəlt ˈkɑːr.bə.neɪt/ |
| Preferred IUPAC name | Cobalt(2+) carbonate |
| Other names |
Spherocobaltite Cobaltous carbonate Cobalt(II) carbonate |
| Pronunciation | /ˈkəʊ.bəlt ˈkɑː.bə.neɪt/ |
| Identifiers | |
| CAS Number | 513-79-1 |
| Beilstein Reference | 3589200 |
| ChEBI | CHEBI:46816 |
| ChEMBL | CHEMBL1201577 |
| ChemSpider | 15215 |
| DrugBank | DB11050 |
| ECHA InfoCard | ECHA InfoCard: 100.013.782 |
| EC Number | 209-644-9 |
| Gmelin Reference | 5698 |
| KEGG | C01773 |
| MeSH | D003054 |
| PubChem CID | 10184947 |
| RTECS number | GF8225000 |
| UNII | LSL33D36UA |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DB11264 |
| CAS Number | 513-79-1 |
| Beilstein Reference | 35355 |
| ChEBI | CHEBI:46816 |
| ChEMBL | CHEMBL1201667 |
| ChemSpider | 20425 |
| DrugBank | DB14537 |
| ECHA InfoCard | ECHA InfoCard: 100.013.807 |
| EC Number | 208-169-4 |
| Gmelin Reference | 12611 |
| KEGG | C01758 |
| MeSH | D003054 |
| PubChem CID | 10100263 |
| RTECS number | FF9650000 |
| UNII | Z3WJA1FI7M |
| UN number | UN3077 |
| Properties | |
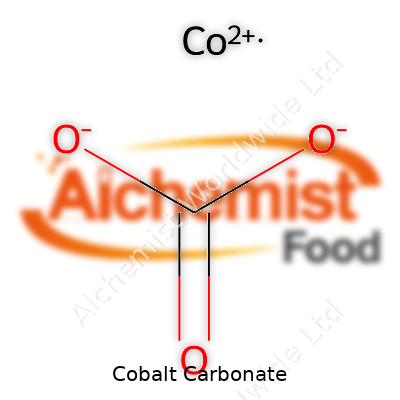
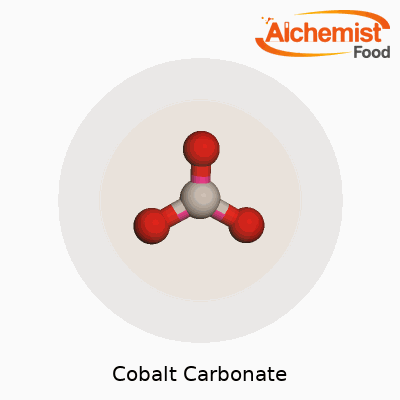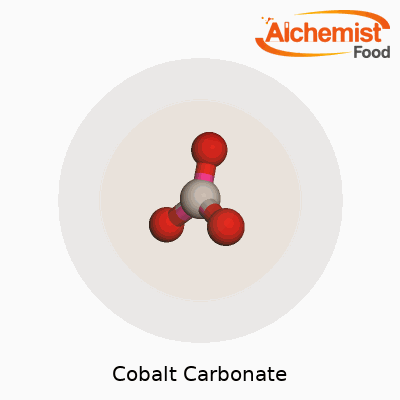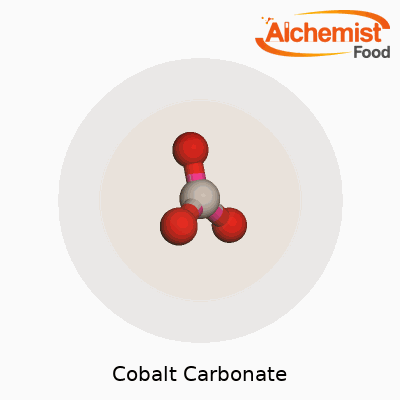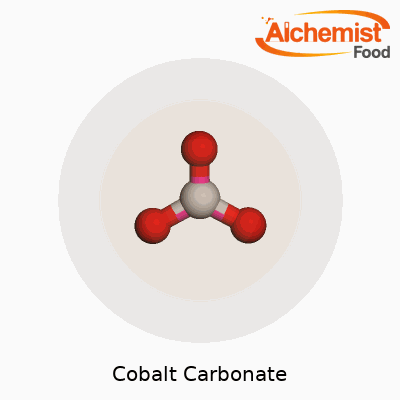
| Chemical formula | CoCO3 |
| Molar mass | 118.94 g/mol |
| Appearance | Pink to Red Violet Powder |
| Odor | Odorless |
| Density | 3.7 g/cm³ |
| Solubility in water | Insoluble |
| log P | -0.47 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.5 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | +2700.0e-6 cm³/mol |
| Dipole moment | 0.0 D |
| Chemical formula | CoCO3 |
| Molar mass | 118.94 g/mol |
| Appearance | Pink to red-violet powder |
| Odor | Odorless |
| Density | 3.7 g/cm³ |
| Solubility in water | Insoluble |
| log P | -2.2 |
| Vapor pressure | Negligible |
| Basicity (pKb) | 8.33 |
| Magnetic susceptibility (χ) | +4120.0e-6 |
| Dipole moment | Zero |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 121.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −547.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −416.4 kJ/mol |
| Std molar entropy (S⦵298) | 110.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -554.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -427.0 kJ/mol |
| Pharmacology | |
| ATC code | V03AB33 |
| ATC code | V03AB56 |
| Hazards | |
| Main hazards | Harmful if swallowed. May cause allergic skin reaction. Suspected of causing cancer. Causes damage to organs through prolonged or repeated exposure. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H317, H319, H334, H335, H341, H350, H360fd, H410 |
| Precautionary statements | P261, P273, P280, P302+P352, P304+P340, P312, P332+P313, P337+P313, P362+P364 |
| Lethal dose or concentration | LD50 (oral, rat): 6,125 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >5000 mg/kg |
| NIOSH | BQ1925000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Cobalt Carbonate: 0.1 mg/m³ |
| REL (Recommended) | 12 mg/m³ |
| IDLH (Immediate danger) | 250 mg Co/m³ |
| Main hazards | Harmful if swallowed. May cause respiratory irritation. Suspected of causing cancer. Causes damage to organs through prolonged or repeated exposure. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H317, H319, H334, H335, H340, H350, H360, H410 |
| Precautionary statements | P260, P264, P272, P280, P302+P352, P304+P340, P308+P313, P314, P362+P364, P405, P501 |
| Lethal dose or concentration | LD50 oral rat 6400 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 7,505 mg/kg |
| NIOSH | WT1925000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Cobalt Carbonate: 0.1 mg/m3 |
| REL (Recommended) | REL (Recommended): 0.05 mg/m³ |
| IDLH (Immediate danger) | 250 mg Co/m³ |
| Related compounds | |
| Related compounds |
Cobalt(II,III) oxide Cobalt(II) oxide Cobalt(II) hydroxide Cobalt(II) chloride Nickel(II) carbonate |
| Related compounds |
Cobalt(II) oxide Cobalt(II) hydroxide Cobalt(II) chloride Cobalt(II) sulfate |

