Cobalt Acetate: A Grounded Look into a Chemical Mainstay
Historical Development
Cobalt acetate traces roots back to the era of early organometallic chemistry. In the 19th century, colorists used cobalt compounds for glass and ceramics. Artisans found cobalt’s vivid blue produced eye-catching pigments, and by late Victorian times, attention shifted to cobalt's soluble forms. Laboratories uncovered cobalt acetate by reacting cobalt metal or basic salts with acetic acid, yielding a vivid pinkish-red powder. It wasn’t just for color: chemists soon realized cobalt acetate offered a reactive platform for catalysis and synthesis. By the mid-20th century, you could spot it in research notes from industrial chemistry labs and university benches around the globe. Demand ballooned with the growth of polyester manufacturing and petrochemical processes during the second half of the 1900s.
Product Overview
Most cobalt acetate commercially available appears as cobalt(II) acetate tetrahydrate, a crystalline powder that stands out for its strong color and ease of dissolution in water. Some forms shift in hue depending on water content, making product quality checks straightforward. Suppliers target various grades: research purity for analytical use, technical for plastics or batteries, and pharmaceutical for specialized catalysts. Large-scale users pay attention to batch consistency, since even trace contaminants can affect catalyst performance or product color.
Physical & Chemical Properties
Cobalt acetate’s main draw comes from its distinctive appearance — a bright rose-red or pink crystalline solid. The tetrahydrate versions melt near 140°C, then decompose, often giving off acetic acid. Thanks to their solubility, these salts form clear, colored solutions in water, alcohol, or acetone. Cobalt’s d-block character means the compound can swap ligands and coordinate with a range of molecules, a property manufacturers use to tune reaction outcomes in industry. Storing cobalt acetate with tightly sealed containers cuts down on moisture uptake and caking, as humid environments speed up clumping and partial hydrolysis.
Technical Specifications & Labeling
Each shipment carries depth in its documentation: assay percentages for cobalt and acetate, limits on iron, copper, and other metals, moisture content, and physical mesh size. Buyers demand these reports to assure compatibility in complex formulations, whether making catalysts, inks, or nutritional additives. Technical labels typically reference the CAS number 6147-53-1 for tetrahydrate, structural formula (Co(CH3COO)2·4H2O), and recognized hazard codes. Labels clearly list supplier, batch, and expiration date, and hazard warnings highlight toxicity and environmental impact. Import controls for cobalt compounds reflect concern over misuse and ensure only legitimately sourced material enters the supply chain.
Preparation Method
Modern production routes start by dissolving cobalt carbonate, oxide, or hydroxide in excess acetic acid at elevated temperature. The reaction forms a pink solution of cobalt acetate, which then gets concentrated under reduced pressure. As water evaporates, the tetrahydrate crystallizes, leaving behind impurities. Factories often repeat recrystallization or filtration to upgrade purity for technical or research markets. All stages require careful control of pH, temperature, and contaminant levels; even slight variations can introduce unwanted byproducts that affect catalyst activity or downstream color results.
Chemical Reactions & Modifications
Cobalt acetate reacts readily with phosphines, diamines, and other ligands — a benefit for synthesizing tailored coordination complexes. Organic chemists often use this salt as a mild oxidant, supporting reactions like the oxidation of alcohols. In industry, cobalt acetate serves as a precursor for cobalt-based catalysts, especially in the production of terephthalic acid. The compound enables oxygen to insert cleanly into benzene rings in liquid-phase catalytic oxidation processes, boosting reaction efficiency. Addition of acetic acid and air helps maintain the catalyst’s activity cycle, so users often recirculate cobalt acetate in closed-loop systems.
Synonyms & Product Names
You'll find cobalt acetate referenced as cobalt(II) acetate, cobaltous acetate, EINECS 204-834-4, or by the moniker “acetic acid, cobalt(2+) salt.” In some literature, names draw from its hydrate state: cobalt acetate tetrahydrate, or just “pink cobalt salt.” International trade documents frequently use these terms interchangeably, and packing lists may show both common and IUPAC names depending on export destination or local chemical registry.
Safety & Operational Standards
Workers handling cobalt acetate need strong protections in place. Cobalt compounds rank among acute inhalation and skin hazards, with risks of sensitization. Chronic exposure connects with heart and lung issues, and studies suggest long-term uptake may increase cancer potential. Best practices in the plant setting call for enclosed handling, vented hoods, gloves, goggles, and strict hygiene protocols. MSDS sheets highlight incompatibilities — strong oxidants, reducing agents, or sources of ignition, given its slight flammability risk combined with organic acid content. Storage areas stay dry and cool, far from acids and bases that could trigger decomposition.
Application Area
Cobalt acetate’s footprint stretches widely across manufacturing and research. Its most impactful use shows up in catalysis for PET (polyethylene terephthalate) production, driving oxidation steps that enable mass-market plastics. The dye and pigment industry taps it for blue and green hues, especially in ceramics or artist’s materials. It lands in batteries as a key precursor for certain cobalt-based cathode materials. Biomedical researchers even experiment with it as a micronutrient for livestock feed or vitamin B12 precursor, although strict dose control is crucial, given cobalt’s toxicity in higher amounts. As industry trends push toward efficient recycling and battery chemistry innovation, interest in cobalt salts remains high.
Research & Development
Publications in top chemistry journals demonstrate an ongoing search for greener, safer synthesis methods. Some labs substitute less hazardous cobalt ores to cut down waste acid effluent. Electrochemical methods, which reduce energy needs and improve selectivity, attract strong academic funding. Industry-backed research targets more active catalyst systems, exploring modifications of the acetate ligand with phosphines or carbenes. In battery research, scientists tweak stoichiometry and drying protocols, looking to lock in precise morphology that boosts charge cycles in lithium-cobalt cells. Interdisciplinary teams model the electronic structure of new cobalt complexes, predicting how subtle changes will affect thermal stability or reactivity in next-generation processes.
Toxicity Research
Cobalt acetate’s health effects spark debate, especially as global demand puts more pressure on extraction and refinement. Animal studies highlight risks of organ damage at relatively low exposure, linking repeated contact to heart, thyroid, and immune system changes. Regular monitoring of industrial workplaces uncovers occasional spikes in airborne cobalt particles, forcing facility redesigns and tighter OSHA limits. Regulatory agencies call for environmental risk assessments, since both acute spills and chronic discharges threaten aquatic life. Analyses of soil and groundwater near old processing sites often detect persistent cobalt, reminding us that responsible manufacturing matters. Scientific reviews call for ongoing long-term studies to track cumulative exposure effects among workers and downstream communities.
Future Prospects
Cobalt acetate faces a shifting landscape shaped by battery technology, recycling goals, and green chemistry mandates. Cleaner supply lines for cobalt ores can cut labor abuses and environmental impact in mining regions. Synthetic labs look for ways to upcycle spent catalysts or extract cobalt from scrap electronics, feeding back into high-purity acetate production. Regulatory agencies set ever tighter exposure thresholds, pushing factories to automate handling and capture every trace of fugitive dust. In the lab, clever ligands and milder syntheses broaden what cobalt acetate can do as a catalyst, while computational modeling sheds light on new molecular architectures. The balance lies in meeting demand for materials science, electronics, and plastics without compromising safety or planet health, and the industry’s next decade looks far from boring.
What are the primary uses of Cobalt Acetate?
Behind the Factory Walls
Cobalt acetate turns up in more places than most folks realize. Steel and glass makers count on cobalt acetate for its strong coloring power. You see the rich blues in glass tiles or pottery glazes—that look comes from cobalt-based compounds. For anyone who's experimented with ceramics, that satisfying deep blue doesn’t show up without a bit of chemistry behind the scenes.
Big chemical plants use cobalt acetate for making catalysts. Catalysts keep processes moving without getting used up, which saves time and money. In the industry known as PET production, used for bottles and food packaging, large reactors use cobalt acetate as an ingredient to speed things up and make the reaction more efficient. Billions of water bottles and food containers only reach their final shape thanks to this step.
Paints, Dyes, and Everyday Goods
You might not see the words "cobalt acetate" on a label in your local hardware store, but the paint on your walls or the wood stain in your toolbox likely owes its life to it. Paint manufacturers include cobalt acetate to help paint dry. In oil-based paints and inks, the compound acts as a drying agent, making tough jobs go faster. DIYers and pros don’t want to wait days for layers to set, especially on humid days.
Textile dyeing makes use of cobalt acetate as well. Some synthetic fibers take on color better when this compound is in the mix. If you’re wearing jeans or using upholstery that holds its look after plenty of washes, cobalt chemistry played a part. While these behind-the-scenes roles don’t grab headlines, they make a difference in the quality and durability of everyday products.
From Batteries to Science Labs
The energy sector has taken a closer look at cobalt acetate in the past decade. Rechargeable batteries, particularly for electric vehicles, rely on cobalt compounds to keep cells stable and deliver more energy. That doesn’t mean all batteries use cobalt acetate directly, but factories create battery-grade materials from it. As the world looks for greener transport options, experts keep searching for ways to recycle or refine cobalt compounds safely.
Research labs also put cobalt acetate to work. In universities and private labs, chemists run experiments with it to develop new catalysts or test reactions for cleaner energy production. Scientists race to extract more value from each gram of cobalt, since mining brings a host of ethical and environmental headaches. Countries with stricter regulations push for more transparency in supply chains and promote recycling tech.
Real-World Health and Safety Concerns
Cobalt acetate doesn’t usually show up in consumer products in pure form, but it demands respect. Anyone who’s worked handling chemicals knows that carelessness leads to trouble. Inhaling dust from cobalt compounds can cause long-term health issues. That’s why factories keep strict controls, use gloves and masks, and train workers for safe handling. Public health guidelines call for routine monitoring, and researchers always look for ways to lower exposure using ventilation systems and protective gear.
Paths Forward: Smarter Use and Safer Practices
It’s not enough to just swap cobalt acetate for something else. Industry relies on its unique blend of properties. What can change is how it’s processed, sourced, and disposed of. Companies invest in recycling setups, reclaiming cobalt from old batteries and factory waste. Environmental groups push for alternatives, but any replacement gets tested for safety and usefulness before major adoption.
As manufacturers and researchers keep blending tradition with new science, cobalt acetate remains a cornerstone in dozens of industries. The challenge is to make its use as responsible as possible—balancing innovation, safety, and sustainability in a world that relies on chemistry far more than meets the eye.
What is the chemical formula and appearance of Cobalt Acetate?
Understanding Cobalt Acetate
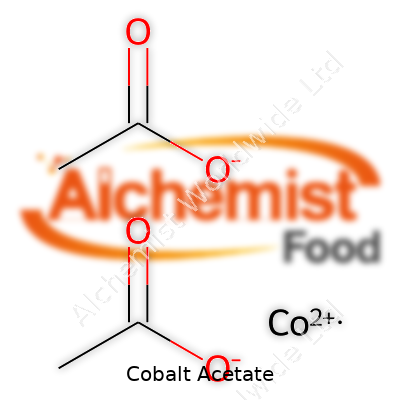
Cobalt acetate grabs attention mostly among chemistry teachers, lab workers, and anyone dealing with industrial catalysts or pigments. With a chemical formula of Co(C2H3O2)2, this compound shows how one metal and vinegar’s key ingredient can come together and create something entirely new. As a hydrated salt, it often appears as Co(C2H3O2)2 · 4H2O, meaning water molecules get caught up in the crystal, changing its look and feel a bit.
Appearance in the Real World
In its common hydrated form, cobalt acetate looks like small crystals or powder, colored rose-pink or reddish-purple. Walking into a lab, it’s hard to ignore the color. Many students, myself included from the early college days, remember the intimidating moment of scooping a pink crystalline powder and double-checking to make sure nothing had been mislabeled. Dry, anhydrous cobalt acetate can appear as a lighter pink to nearly lavender, though most chemists won’t see it in this stricter form unless they’re taking extra caution to remove moisture.
The color depends on hydration because water changes the electronic environment around cobalt ions. Jewelers or artists interested in ceramic glazes sometimes use the visual quality of cobalt compounds without realizing how the chemistry tricks the eye. The hydrated type remains the standard for most lab and industrial uses because it’s stable, stores well, and dissolves easily in water, so mixing for reactions or setting up for experiments is less hassle.
Role in Industry and Science Labs
The rosy powder of cobalt acetate sits on countless shelves, not just as a curiosity. Paint companies rely on its unique color and chemical properties to create blues, pinks, and purples in ceramics or glass. It plays a central part in drying agents for paints and varnishes, helping coatings cure faster. Chemical engineers and researchers use it to make catalysts for producing plastics and even some renewable energy fuels. Time in a university lab taught me that cobalt acetate ends up in surprising places, acting as a middle step or helper molecule in reactions that end up in fuel cells or pharmaceuticals.
What many forget, cobalt compounds don't come without warning labels. The vibrant color can be deceiving—like other cobalt chemicals, they need careful handling. Prolonged or repeated exposure to dust may irritate skin and eyes and, over time, could harm the lungs. That said, following standard safety basics (gloves, mask, working under a hood) drastically cuts risk, and most labs have this well in hand after years of practice and awareness training.
Importance of Recognizing Appearance and Properties
Spotting cobalt acetate on a shelf isn’t just a chemistry quiz. In my lab experience, color and crystal shape give the first hint whether a reagent is fresh, pure, or contaminated. Any unexpected change—dull color, powder clumping, moisture inside a sealed bottle—means it’s time for a closer look, maybe even a full test.
For startups getting into battery or pigment development, it pays to source cobalt acetate that matches visual cues and not just a label or datasheet. Suppliers with proven records and open safety information create peace of mind that raw materials won’t derail safe, effective product development. Educators can use its bright appearance and chemical versatility to spark curiosity in younger students and make abstract science hands-on and visual.
Is Cobalt Acetate hazardous to health or the environment?
The Real Concerns: Understanding Exposure
Anyone who has worked around industrial chemicals knows this: safety goggles and gloves are not fashion accessories. Cobalt acetate pops up in places like paint dryers, catalysts, and pigments. It’s a pinkish powder, easy to breathe in or get on skin. Direct contact with this compound brings real risks. I once helped clear out a chemical storeroom and wore a mask specifically because the material safety data sheet screamed about respiratory irritation and skin rashes. The powder doesn’t play nice with lungs or skin. Over time, inhaling it can set off asthma-like symptoms and trigger allergies. Regular contact ups the chance for chronic heart and thyroid issues, too. I learned fast that it stains your hands deep pink and, if you ignore the stinging, leaves them cracked and dry by the end of the shift.
There’s no question cobalt acetate can be toxic. If someone swallows even a small amount, nausea and diarrhea often follow. At higher doses, the kidneys and heart start to suffer. Health agencies—think OSHA and NIOSH—place strict exposure limits on cobalt compounds for these reasons. Even low-level exposure raises red flags if it’s day-in, day-out. Chronic, low-level exposure to cobalt compounds links to hard metal lung disease, a form of scarring in lung tissue. Some working with cobalt compounds have developed sensitivity, where exposure triggers rashes, itching, or even asthma attacks. This isn’t rare. It shows up often enough that factory managers keep company doctors on speed dial.
Environmental Fate: Where It Ends Up Matters
Disposal isn’t simple. Cobalt acetate, once it leaves a factory, doesn’t vanish quietly. Water pollution turns real fast with improper disposal, as cobalt makes its way into rivers and streams. Fish and small aquatic life soak up heavy metals with nowhere for it to go, leading to bioaccumulation. I’ve read research out of mining towns where runoff stained whole ponds pink. Amphibians and fish disappeared. Even trace metal disrupts food chains. And this isn’t about distant industrial sites; some small towns have seen wells and soil tested high for cobalt, forcing advisories against gardening and fishing local rivers.
Plants take up cobalt easily. Too much in soil stunts their growth or kills them outright. Excess cobalt changes the nutrient balance. Some crops stop producing fruit, others show yellow, wilted leaves. If livestock eat grass from contaminated soil, the metals collect in organs and milk, which affects human diets down the line. Years ago, farmers in a cobalt-rich region complained about cows refusing pasture. No one paid attention until veterinary reports revealed cobalt toxicity. It made headlines for months; folks demanded tighter supervision and cleanup efforts.
Better Practices and Protecting Ourselves
Personal experience in industrial settings shows real progress comes from common sense and following rules. Regular monitoring beats relying on alarms. Good ventilation, protective gear, and clear handling instructions make a difference in exposure numbers. Industrial hygiene programs where people actually speak up about unsafe conditions lead to changes. One shift supervisor at my old plant set up peer monitoring—folks checked each other’s safety gear before handling cobalt acetate. Safety culture improved, and health clinic visits dropped.
Improved waste treatment and zero-discharge policies help, but oversight matters. Regulators need transparency from companies in reporting spills and disposal methods. Simple steps like enforcing proper labeling, spill plans, and air filtration keep cobalt acetate from moving offsite and harming communities. Grassroots involvement—neighbors and workers united in demanding testing and reporting—keeps pressure on industries to use best practices, not just what’s legally required. We learn from the places where cobalt pollution made folks sick or wiped out local fish. Ignoring the hazards or treating them as someone else’s problem guarantees a repeat. Our choices matter, at work, at home, and in our towns.
How should Cobalt Acetate be stored and handled safely?
What Makes Cobalt Acetate a Topic Worth Discussing?
People working around chemicals weigh the risks every day. Cobalt acetate usually pops up in labs or factories. The concern isn’t just for chemists—it affects janitors, warehouse workers, and anyone moving barrels or bags through hallways. Cobalt compounds have a role in batteries and pigments, but where safety falters, health pays the price. Handling cobalt acetate carelessly can cause skin rashes, breathing problems, and—over the long haul—even cancer. Research from agencies like the International Agency for Research on Cancer keeps raising red flags about toxic metal exposure.
Simple Storage Habits Save Trouble
Dust, leaks, and absent-minded moments start many chemical accidents, not catastrophic explosions. A bag torn open on a shelf, a leaky drum, or poor labeling lets dust float into the workspace. It only takes a little on your skin or the wrong inhale for cobalt acetate to cause trouble. Keeping it in sealed, labeled containers stops most headaches before they start. I’ve seen coworkers skip this, and cleaning up powdery blue dust never gets easier.
Storage spots matter. Humidity and sunlight often damage packaging, and even stable solids like cobalt acetate might break down over time if stored poorly. Tucked away in a designated chemical cabinet—preferably with a tray underneath—containers stay right where they are supposed to. Fire inspectors often call out warehouses after finding oxidizers and flammable chemicals stacked together. Cobalt acetate might not start a fire, but it doesn’t belong by heat sources or reactive chemicals.
Personal Risk Grows With Every Shortcut
Complacency builds over time. I’ve seen experienced staff skip gloves, hoping to shave minutes off their day. But cobalt acetate dust on sweaty skin sticks like talc. Once absorbed, cobalt affects the heart and lungs. The CDC reminds workers about wearing nitrile gloves, goggles, and dust masks. This is not just for the sake of rulebooks—catching a cough in a dusty corner or feeling your hands itch stings long after a shortcut taken.
Work surfaces absorb spills and dust by the end of the shift. Wet wiping, not dry sweeping, locks powder down before it drifts into the air. Used paper towels need immediate disposal in marked hazardous waste bins. Regular cleaning at the end of each shift can mean the difference between a safe lab and a hidden health problem.
Training Makes Safe Handling Habitual
Rules on paper don’t make anyone safer unless people follow them. Briefings on what cobalt acetate does to the body drive the point home. I once saw a young tech skip paperwork training, then mistakenly pour the wrong waste down a sink. That led to an expensive shutdown and an EPA visit nobody wants to repeat. Frequent, honest conversations help everyone remember that this is not just another bland powder.
Spill kits, eye wash stations, and regular reviews of emergency procedures make storage and handling more forgiving when things go sideways. Good training starts with clear lists of do’s and don’ts and real-world stories—lessons people remember, rather than dry instruction.
Lasting Safety Comes From Everyday Choices
What starts as a low priority can spiral fast. Cobalt acetate won’t get safer because people ignore it. Every sealed lid, every glove, and every label puts a layer between risk and daily routine. Safe habits are easier to teach than regrets are to explain.
What is the shelf life and recommended storage conditions for Cobalt Acetate?
Storing Cobalt Acetate for Safety and Performance
Cobalt acetate finds regular use in chemistry labs, battery research, and even textile dyestuffs. This little blue compound plays a bigger role than most folks might guess. The thing is, it reacts to the world around it — air, moisture, and anything that creeps into an open container. Left overlooked, it can clump, degrade, or take up water. Quality changes, and the data from your beakers or reactors takes a hit.
Real Shelf Life: Not Just a Number
Talk to anyone who has run a well-managed lab, and you’ll hear the same general scoop. Unopened cobalt acetate, sealed tight and kept dry, holds up for about two years without much worry. Opened containers, on the other hand, see shifts in quality after one year. I’ve watched teams mark the date of opening and check weight or appearance every quarter. Dull color, caking, or moisture beads on the inside of the jar—these small changes can mess with dosing in sensitive reactions.
Keeping It Dry and Secure
Humidity stands as the biggest enemy. Cobalt acetate grabs water from air, so a dry storeroom pays off in every way. Most facilities stash it in tightly sealed containers, sometimes under nitrogen if humidity swings in the building. Silica gel packs work well for short-term stockpiles. We kept ours in amber glass or HDPE containers, far from acids or metals that could spark unwanted reactions or stains. A silica gel packet costs a few cents but saves dollars on spoiled material.
Avoiding Temperature Surprises
Room temperature — 20 to 25°C — fits best for storage. Lab refrigerators can slow water absorption, but unless you live in the tropics, cool and steady beats cold and damp. I learned early on: the back of a bench near a sunny window shortens shelf life quickly. Direct sunlight cooks bottles, and plastic lids loosen just enough for air to sneak in. I once opened a bottle kept near a window; crystals had fused and the color went off, showing clear signs of breakdown.
Labeling Adds a Layer of Security
Good habits serve well here. Clean gloves before scooping out any powder, label each batch with purchase and open dates, and write down odd changes in color or texture. This kind of record-keeping takes seconds, but it builds trust in your sample reliability. There’s nothing worse than troubleshooting a failed experiment and realizing months later that the culprit was a compromised bottle from the shelf.
What Science and Regulations Say
Health and safety rules back up these basic steps. Material Safety Data Sheets highlight the need for tightly closed storage and warn about inhaling or swallowing dust. Long-term exposure brings occupational risk, which shows why frequent checks matter for industry and research. Linked data from Sigma Aldrich and the NIOSH database confirm that cobalt acetate does not last forever, even when untouched. Those facts match the daily reality in every stockroom or warehouse shelf.
Better Practices, Better Results
Set a reminder to check old inventory, rotate your stock, and don’t be shy about discarding what looks even a hair off. Losses from spoiled batches can far outweigh the price of ordering fresh. Well-stored cobalt acetate supports accuracy, safety, and saves money for both small labs and big manufacturers—the difference often comes down to details noticed by careful eyes and well-trained hands.
| Names | |
| Preferred IUPAC name | cobalt(II) acetate |
| Other names |
Cobalt(II) acetate Acetic acid cobalt(2+) salt Cobaltous acetate |
| Pronunciation | /ˈkoʊ.bəlt ˈæs.ɪ.teɪt/ |
| Preferred IUPAC name | Dioxido(dioxoacetato)tetrahydroxycobalt |
| Other names |
Cobalt(II) acetate Cobaltous acetate Acetic acid cobalt(2+) salt Cobalt diacetate Acetate de cobalt |
| Pronunciation | /ˈkoʊ.bɔːlt ˈæs.ɪ.teɪt/ |
| Identifiers | |
| CAS Number | 71-48-7 |
| 3D model (JSmol) | JSmol model string for Cobalt Acetate: `C[C@@H](=O)O.Co` |
| Beilstein Reference | 1719591 |
| ChEBI | CHEBI:35274 |
| ChEMBL | CHEMBL1200387 |
| ChemSpider | 12109 |
| DrugBank | DB11364 |
| ECHA InfoCard | 100.031.421 |
| EC Number | 200-755-8 |
| Gmelin Reference | 763 |
| KEGG | C00814 |
| MeSH | D003053 |
| PubChem CID | 3034164 |
| RTECS number | AG7350000 |
| UNII | 7BXA1IHZ1W |
| UN number | UN3288 |
| CAS Number | 71-48-7 |
| Beilstein Reference | 1691076 |
| ChEBI | CHEBI:35202 |
| ChEMBL | CHEMBL1233569 |
| ChemSpider | 8085 |
| DrugBank | DB11363 |
| ECHA InfoCard | 100.004.259 |
| EC Number | 200-755-8 |
| Gmelin Reference | 6741 |
| KEGG | C00933 |
| MeSH | D003059 |
| PubChem CID | 3033968 |
| RTECS number | AG4375000 |
| UNII | NF9M928H7F |
| UN number | UN3283 |
| Properties | |
| Chemical formula | Co(CH₃COO)₂ |
| Molar mass | 177.03 g/mol |
| Appearance | Pink crystalline solid |
| Odor | faintly acetic |
| Density | 1.7 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.037 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 8.47 |
| Magnetic susceptibility (χ) | +1380×10⁻⁶ |
| Refractive index (nD) | 1.547 |
| Dipole moment | 4.24 D |
| Chemical formula | Co(C2H3O2)2 |
| Molar mass | 177.07 g/mol |
| Appearance | Pinkish crystalline solid |
| Odor | slight vinegar odor |
| Density | 1.7 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.2 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 8.22 |
| Magnetic susceptibility (χ) | +227×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.542 |
| Dipole moment | 4.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 155.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -829.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1687 kJ/mol |
| Std molar entropy (S⦵298) | 155.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -859.3 kJ/mol |
| Pharmacology | |
| ATC code | V03AE09 |
| ATC code | V09XX06 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause an allergic skin reaction, may cause cancer, suspected of damaging fertility or the unborn child, toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H317, H319, H334, H341, H350, H360, H372, H410 |
| Precautionary statements | P210, P261, P280, P302+P352, P305+P351+P338, P308+P313, P501 |
| NFPA 704 (fire diamond) | 3-2-0 |
| Flash point | > 210 °C (410 °F) |
| Autoignition temperature | 480°C |
| Lethal dose or concentration | LD50 oral rat 681 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 3,506 mg/kg |
| NIOSH | MH0350000 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | REL (Recommended Exposure Limit) of Cobalt Acetate is "0.05 mg/m³ (as Co), time-weighted average (TWA) |
| IDLH (Immediate danger) | 250 mg/m3 |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; may cause cancer; suspected of causing genetic defects; may cause damage to organs through prolonged or repeated exposure |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H317, H332, H334, H350, H410 |
| Precautionary statements | P210, P261, P273, P280, P302+P352, P304+P340, P305+P351+P338, P308+P313, P314, P405, P501 |
| Lethal dose or concentration | LD50 (oral, rat): 271 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 6,068 mg/kg |
| NIOSH | AJ3325000 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | 0.02 mg/m³ |
| IDLH (Immediate danger) | 250 mg/m³ |
| Related compounds | |
| Related compounds |
Cobalt(II) chloride Cobalt(II) nitrate Cobalt(II) carbonate Cobalt(II) oxide |
| Related compounds |
Cobalt(II) chloride Cobalt(II) nitrate Cobalt(II) carbonate Cobalt(II) sulfate |

