Citric Acid Monohydrate: A Deep Dive into History, Science, and Use
Historical Development
My first kitchen encounter with citric acid came years ago, adding a sour punch to a homemade jam. What I didn’t know then is that people have leaned on this compound for ages. In the late 1700s, Carl Wilhelm Scheele isolated citric acid from lemon juice, turning a tangy kitchen staple into a serious science subject. Within a century, the world shifted from squeezing barrels of fruit to producing citric acid on an industrial scale, mainly using mold fermentation. This jump transformed a pantry ingredient into a global commodity for food, medicine, and cleaning.
Product Overview
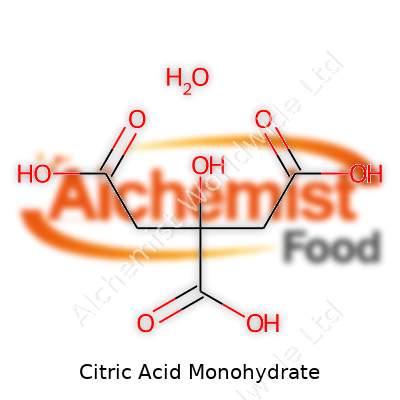
Every time someone sprinkles citric acid into a recipe or a cleaning solution, they’re working with a white, crystalline powder that looks a lot like sugar. It's not fancy and doesn’t call much attention to itself, but it has a sharp, tart flavor and dissolves easily in water. Chemically, it goes by C6H8O7·H2O, which just means one molecule of water tags along with each molecule of citric acid in the monohydrate version. That hydrate part affects its handling, since the powder can cake if it sits too long in a humid room. Still, its simple structure lets it mix well in food, drinks, and countless industrial products.
Physical & Chemical Properties
Grab a scoop of citric acid monohydrate and you’ll notice its fine, almost silky texture. It melts at about 100°C, but above that you’re left with the anhydrous (water-free) version. It’s one of those rare acids that’s both safe to taste and able to bump the pH in solutions, making it popular for both flavor and chemical adjustment. At room temperature, it dissolves fast, so beverage makers and home canners love it for consistent results. On the chemical side, citric acid stands out because it has three carboxyl groups—meaning it can grab onto metals, form chelates, and break down various stains and deposits.
Technical Specifications & Labeling
Citric acid monohydrate usually enters the market with tight purity standards. Regulatory bodies, including the Food Chemicals Codex and European Pharmacopeia, control everything from residual heavy metals to ash content. Labels list it as food-grade or pharmaceutical-grade, with a purity topping 99.5%. Whether found in a one-ton bulk sack or a five-gram sachet, labeling calls out its acid strength, batch number, and best-by date for both safety and traceability. Food and drug rules require the full chemical name and the recommended storage conditions. The packaging changes depending on the end-use, but information transparency remains the same across all sectors.
Preparation Method
Modern production skips the lemon grove and heads straight to fermenters. The most reliable manufacturers use a mold called Aspergillus niger, which munches on sugar solutions—often from corn, sugar beet or molasses—under controlled temperature and oxygen levels. The fermentation gives off citric acid as a byproduct, and technicians use lime and sulfuric acid to pull it from the broth and then purify it into crystals. This method scales easily, slashes costs, and increases purity way beyond what’s possible by pressing fruit. The process left a huge mark on world trade, turning this once-expensive extract into a workhorse for manufacturing, health, and food companies everywhere.
Chemical Reactions & Modifications
Citric acid has a reputation as a chelator, breaking up metal buildup or tank deposits by clinging to stray ions. Bonus: it won’t damage piping or leave a dangerous residue. On a basic level, it reacts with alkalis to make citrate salts, which show up in everything from workout supplements to fizzing bath bombs. Heating citric acid with certain alcohols creates esters—chemicals with fruity scents for perfumes and flavors. By combining it with potassium or sodium bases, manufacturers get buffers for controlling pH in technical processes or pharmaceuticals. Its flexibility in both home and industrial chemistry comes from its three-point structure, which lets it join multiple partners in the lab or production line.
Synonyms & Product Names
Most chemical supply catalogs list citric acid monohydrate by its official name, but you’ll also find it under water of hydration, 2-hydroxy-1,2,3-propanetricarboxylic acid monohydrate, or just plain “sour salt.” Some brands call it “food acid 330,” tying in with food additive coding. International suppliers use “acide citrique monohydraté” in French or “Acido Citrico Monoidrato” in Italian. While the names change, the physical substance remains the same—a trusted ingredient across cultures and systems.
Safety & Operational Standards
I’ve handled citric acid without gloves many times, but safety data always urge respect. In the workplace, it can irritate skin, eyes, or lungs if handled carelessly, especially as powder. Best practices call for gloves, eyewear, and dust-control in industrial settings. Citric acid doesn’t ignite under normal conditions, but mixing it with strong oxidizers or bases can trigger violent reactions. Regulatory agencies like OSHA and the European Chemicals Agency list it as low-hazard, but enforce ventilation and spill management protocols. On the food side, manufacturers meet standards from groups like the FDA and EFSA, proving each batch’s purity and absence of unsafe residues. Consistent employee training and proper ventilation in plant environments offer the most practical risk reduction.
Application Area
Citric acid monohydrate’s reach feels endless. Home cooks turn to it for canning, where it sharpens flavor and helps keep bacteria at bay. The food industry scales up, using it to adjust tartness, stabilize powders, and prevent fruit from browning on supermarket shelves. Drink companies count on citric acid for the snap in sodas and candies. In cleaning, its natural low-toxicity breaks down hard-water scale and rust on glass, metal, and ceramics, all without a harsh chemical smell. The pharmaceutical world trusts it for effervescent tablets and as a buffer in injectable medicines. Even water treatment plants value its non-toxic strength for flushing pipes and dissolving iron or calcium. Whoever needs a mild, edible acid with reliable performance can count on this compound.
Research & Development
Research teams keep probing the boundaries of citric acid monohydrate. There’s always a push for better fermentation yields, tapping into new substrates like agricultural waste to cut costs and boost sustainability. Researchers study modified citric acids for drug delivery or as biodegradable agents in packaging films. Scientists analyze how to tweak its molecular backbone for stronger metal chelation in environmental cleanup. A popular focus lies in pairing citric acid with green technologies, such as using it in eco-friendly detergents and water treatments. Even in food science, teams look for new flavor-pairing options and shelf-life stabilization methods built around the predictable qualities of citric acid.
Toxicity Research
Most people meet citric acid in lemonade, so it has a safe reputation. At high doses, though, it can irritate the digestive system or skin. Long-term research in animals and humans shows low toxicity—citric acid breaks down easily in the body and gets recycled through metabolic pathways. Regulatory studies document that allergic reactions are rare, but some workers may develop skin irritation after prolonged contact. Environmental studies check that, after disposal, citric acid rapidly biodegrades and doesn’t linger or poison waterways. Food safety agencies cap allowable daily intake to avoid minor digestive upset, but in practical use both short- and long-term risks stay low.
Future Prospects
Looking ahead, demand for citric acid monohydrate keeps growing with the world’s appetite for processed foods, wellness supplements, and green cleaners. The chemical industry will need smarter, cleaner fermentation processes tapping into bio-waste instead of food crops, protecting both margins and the environment. Biochemists keep exploring novel citric acid compounds to make medicines safer or more effective. Researchers in sustainable agriculture investigate citric acid as a soil amendment for micronutrient release. The drive toward health-conscious consumer products promises to keep citric acid front and center, with new applications in everything from vegan meat textures to plant-based plastic films. Strong research and ethical production standards will pave the way.
What is Citric Acid Monohydrate used for?
Everyday Encounters With Citric Acid Monohydrate
Anyone who has picked up a can of soda or read the ingredients on a bag of sour candy has come across citric acid monohydrate. This powder gets its name from citrus fruits, mostly lemons and limes. You find it holding a steady place in home kitchens, laboratories, food production, and even cleaning closets. Behind every sour taste or fizz in packaged drinks, there's a good chance citric acid monohydrate is involved.
A Vital Ingredient in Food and Drink
Food and drinks rely on this powder for its ability to sharpen flavors and keep things fresh. In jams, candies, or soft drinks, it serves to boost sourness and balance out sweetness. Safety comes as another reason for its popularity. Bacteria don’t do well in acidic environments. Citric acid drops the pH of its environment, slowing spoilage. From my own kitchen, adding a pinch to home-canned tomatoes keeps their color and taste bright through the winter months.
Processed foods often contain citric acid monohydrate not only for flavor but as a preservative. According to the FDA, it has been tested and used in food for decades and is considered safe when consumed in reasonable amounts. It also works as an emulsifier in cheese-making, giving that smooth, creamy consistency shoppers look for.
A Clean Cut in Household and Industrial Cleaning
Look under your sink or in your favorite local store. You’ll spot citric acid in more than a few cleaning products. The powers at work here relate back to its acidity. Hard water leaves behind chalky limescale deposits; citric acid dissolves these with little effort. Glass cleaners and bathroom sprays often include it, helping tackle soap scum and mineral rings on fixtures.
As someone who’s tried dozens of “natural” cleaning tricks, a simple slurry of citric acid and warm water scrubs my kettle clean, beating fancier store-bought options. For industrial uses, factories favor it for cleaning complicated machinery, where harsh chemicals would damage surfaces or harm workers.
Helping Out in Medicine and Pharmacy
Beyond flavor and cleaning, citric acid plays a quiet part in hospitals and pharmacies. It appears in medicines as a stabilizer, ensuring active ingredients maintain their power. Some powders or tablets need to fizz in water for easier swallowing. Citric acid teams up with baking soda to give that familiar bubbling action, helping make supplements palatable for kids and adults who struggle with swallowing pills.
Pharmacists appreciate how it helps with pH balancing. Certain drugs need a specific acidity to work as intended, especially in IV drips or syrups for children.
Building a Safer and Greener Future
This ingredient stands as proof that not every chemical name should cause worry. Sourced mostly from fermentation using non-toxic strains of Aspergillus niger, citric acid supports sustainability in manufacturing because it comes from renewable resources. Instead of petrochemical origins, this path keeps it approachable and cost-effective, making it popular with both household brands and eco-friendly startups.
Moving beyond the kitchen and cleaning aisle, its gentle nature encourages more companies to swap out harsh, synthetic options in favor of safer, naturally-derived products. With more research and advocacy, consumers and businesses can both enjoy effective cleaning, preservation, and improved taste without harmful byproducts. A closer look at citric acid monohydrate shows everyday chemistry building better homes, tastier food, and a cleaner world with just a pinch of powder.
Is Citric Acid Monohydrate safe for consumption?
What Is Citric Acid Monohydrate?
Citric acid monohydrate shows up in more foods and drinks than many realize. Grocery store shelves hold items laced with it, from sodas to canned tomatoes to candies. It's the stuff that gives sour candies their tang and keeps jams shelf-stable a little longer. At its core, citric acid comes from citrus fruits like lemons and limes, but most of what we see in food manufacture gets produced by fermenting sugars with a particular strain of fungus, Aspergillus niger. Adding water molecules creates the “monohydrate” version—basically a more stable, powdered form that handles shipping and blending well.
Scientific Community Stance on Safety
Scientists and food safety regulators around the world have studied citric acid for decades. Agencies like the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) have stamped it as safe for regular consumption. Their decision isn’t based on guesswork. Long-term animal studies and examinations of food chemistry help rule out toxic effects. Citric acid heads the GRAS (Generally Recognized As Safe) list in the United States, which means food makers rely on it without needing a warning label or special permit.
Potential Concerns
Online rumor mills sometimes buzz about the dangers of additives like citric acid monohydrate. Personal experiences fuel these stories—one person gets a stomach upset, another notices skin irritation after eating certain processed foods. These cases exist, but large population studies haven’t turned up evidence that would justify pulling citric acid from kitchens or supermarket shelves. Some people with rare sensitivities or allergies to mold might notice problems. Anyone living with this risk often pays close attention to food labels and knows what to avoid.
Role in Food and Health
As someone who reads nutrition labels and cooks at home, I see citric acid as a practical preservative and flavor booster. Home canning books even suggest a dash of it to increase the acidity of tomatoes for safe storage, reducing the risk of harmful bacteria like Clostridium botulinum. In high concentrations, citric acid can irritate the mouth—think of eating too many sour candies in one sitting—but the amounts added to foods don’t cause damage under normal circumstances. Dentists do remind parents that eating sour candies too often can erode tooth enamel, but this has more to do with acid exposure in general than anything specific to citric acid itself.
Manufacturing and Additive Sources
Most citric acid monohydrate available for commercial use comes from fermented sugar with the Aspergillus mold. This usually means corn or sugar beets, not directly from citrus fruits. The process removes the source material so thoroughly that safety organizations consider the additive vegan and kosher, suitable for all sorts of diets. Some worry about genetic modification if corn supplies get used, but the finished citric acid doesn’t carry DNA from the original plant.
Suggestions for Concerned Consumers
For those who want to avoid all additives, it helps to focus on whole foods—fresh fruit, vegetables, grains, and meats. Cooking from scratch means full control over what goes into each meal. Anyone worried about potential allergies could keep a food diary, jotting down symptoms and matching them to what gets eaten. If uncertainty lingers, talking with a doctor or a registered dietitian can clear doubts with tailored advice. For most people, citric acid monohydrate keeps food safe and fresh, and current research supports its safety.
How should Citric Acid Monohydrate be stored?
Why Storage Matters for Citric Acid Monohydrate
Citric acid monohydrate might look harmless at first glance—a bag of harmless white crystals, found in everything from fizzy drinks to cleaning solutions. Still, improper storage can turn it into a problem. Keeping it stable isn’t just about product quality. Poor storage invites contamination, loss of potency, or even risks like accidental ingestion. My work in a small-batch food manufacturing space showed how skipping the basics—like sealing up opened containers—leads to wasted supplies and serious messes.
Keep It Dry: The Enemy Is Moisture
Water clings to citric acid monohydrate. Exposure to humidity causes the powder to clump and harden, making it harder to use and less effective. It also attracts contaminants faster than you’d expect. According to the Food Chemicals Codex, damp conditions will speed up spoilage and reduce shelf life. I’ve seen a misplaced scoop, wet from washing, ruin an entire bag during a summer heatwave. The answer is a tight lid—best with a screw cap—stored someplace low in moisture. Many storage mishaps come from plastic bags or loosely covered boxes. Use sealed containers made of plastic or glass, and choose somewhere off the ground, away from drains and cleaning stations.
Heat Can Change Everything
Heat makes citric acid lose its monohydrate water, shifting the chemical balance. Even if you aren’t running a commercial-grade operation, stashing it next to hot appliances or in a sunlit storeroom causes problems. In a restaurant stockroom, I once saw boxes slump near the kitchen oven vent; after a month, what came out of those boxes was almost unusable. The science backs this up—the Handbook of Food Additives recommends cool, shaded storage, far from heat sources. Room temperature works in most climates, but anywhere over 25°C, risk increases. Don’t stick it on top of the fridge, and skip the shelf above the radiators.
Separate Storage: Why It Matters
Citric acid monohydrate often shares warehouse space with other foodstuffs or chemicals. I’ve watched cross-contamination happen in crowded pantries. Store it away from anything volatile, strongly alkaline, or dusty. It reacts with metals and alkalis; storing it next to boxes of baking soda or near bleach can cause unsafe fume build-up and spoil the batch. Metal shelves with epoxy coating or plastic racks work better than raw steel to avoid rust spots or unwanted chemical reactions.
Keep Out of Reach—Label and Safe Access
Children, pets, and untrained staff reach for whatever sits at eye level. A sharp label helps everyone. For my team, I once used color-coded bins, but even a simple sticker in a bold color keeps confusion low. Always include the product name and a clear warning. Make sure access is limited, either by a latch, a locked cabinet, or a dedicated storeroom.
Regular Checks to Avoid Surprises
Simple routines keep things in order. Every month, pull the container, look for leaks, and check for caking or weird colors. Rotate stock so older citric acid gets used before a new bag opens. This approach borrowed from the grocery business helps cut down on unnecessary waste and keeps quality consistent.
Final Thoughts From the Workplace
I’ve handled citric acid monohydrate both at home and in production settings over the years. Some headaches—like moisture sneaking in or heat creeping up on a shelf—are easy to avoid with basic habits. It’s not expensive, and it doesn’t take up much space, but letting storage slide causes headaches that smart planning prevents. When in doubt, act like the bag will matter to your end use, whether for lemonade or lab work.
What is the difference between Citric Acid Monohydrate and anhydrous citric acid?
A Look at Two Common Kitchen and Lab Ingredients
Citric acid turns up everywhere, from fizzy drinks and jelly sweets to your bathroom cleaning sprays. Talking to food science students, home cooks, and DIY chemists over the years, I’ve seen a lot of confusion about which type of citric acid really matters for what purpose: monohydrate or anhydrous. The names sound technical, but if you work with ingredients, the distinction comes down to water content, how that affects storage, measuring, and everyday applications.
What’s the Real Difference?
Monohydrate citric acid comes with water built into its crystals. Scoop up a spoonful, and about ten percent of its weight is water—even though it looks and feels like a white powder. Anhydrous means dry. Crystals of this type have no water attached. Both act the same way to add sourness or regulate acidity, but people notice differences when they measure by weight or need the acid to dissolve fast.
Where It Matters Most
Bakers and home canners sometimes reach for whichever jar shows up first in the pantry. In recipes that rely on grams or ounces, monohydrate tips the scale a little heavier than anhydrous for the same tart effect. Over the years, I’ve watched folks end up with strawberry jam that tastes odd or batches of bath bombs that never fizz right. Most hobbyists don’t realize that swapping one type for the other can throw the balance off. If you’re trying to follow a standard recipe to the letter, it’s worth checking the label closely.
Industries pick one or the other based on shelf stability and how well it dissolves. My relatives in dry States—or in homes with hot, humid kitchens—often found anhydrous grains clumping less than the hydrated version. Anhydrous citric acid keeps longer on the shelf. Small labs dealing with precise titrations and pharma companies go for anhydrous powder when water content must not stray, because even a hint can throw off sensitive measurements.
The Science: Not Just Water Weight
With monohydrate, water molecules are stuck right into the crystal, which means it melts a bit slower and sometimes needs more heat or agitation. People making soda syrups or syrups for snow cones notice that not all crystals dissolve at the same rate. In my home kitchen, when I dashed in a hefty scoop of the monohydrate thinking the effect would be the same, my syrup ended up a bit gritty. The anhydrous crystals, by contrast, went clear much faster.
That trace of moisture in monohydrate can sometimes help with storage in dry climates but turns into a problem in humid weather. Over the years, enough hobby crafters left storage jars open too long only to find clumps that wouldn’t blend smoothly into their bath salts and cleansers.
Better Choices, Fewer Mistakes
Choosing the right type ties back to knowing your purpose and paying attention to the little details on labels. For recipes in grams—especially those written for the food industry—anhydrous offers consistency and longer shelf life. In home settings with less fussing over precision, both work, but substituting one for the other asks for a calculator or a handy conversion chart. Monohydrate weighs in at about 109 grams for every 100 grams of anhydrous if you want to keep the acidity balanced.
Fact: The U.S. FDA recognizes both forms as safe for use in foods and pharmaceuticals, but dosing matters. Published test studies show that humidity, temperature, and handling affect the shelf life and purity—something I learned firsthand after too many sticky bags and failed projects.Avoiding Everyday Pitfalls
A little bit of label reading and a scoop on the kitchen scale go a long way. In my experience, starting with the right type, storing powders airtight, and double-checking amounts make all the difference. Small steps cut down on wasted food, ruined recipes, and unpredictable reactions—whether you're making lemonade, preserving fruit, or mixing up science projects at home.
What is the shelf life of Citric Acid Monohydrate?
Understanding the Real-World Impact
Citric acid monohydrate shows up in my kitchen, on the factory floor, and in school science labs. Its reputation as a safe, food-friendly acid lands it in sodas, canned goods, cosmetics, bath bombs, and even cleaning sprays. Any time a bag of white crystals sits on the shelf long enough, someone asks: how long will it last? This question isn’t just for chemists or manufacturers. Home cooks, teachers, and small business owners all want straight answers on storage — and what it means for safety, cost, and waste.
What Science Says About Its Longevity
Pure citric acid monohydrate keeps its structure for years under normal storage. Most suppliers stamp a shelf life between three and five years from the date of manufacture, tracing this confidence back to scientific stability data. Given correct storage — sealed container, staying dry, room temperature — citric acid won’t support bacteria or mold. Its very purpose in food preservation comes from this same resistance to spoilage.
With experience in ingredient purchasing, I see that well-kept citric acid outlasts its label date. Sometimes bulk batches keep a clean texture and sour punch well past the expiration printed. The crystal’s low moisture content also slows down any chemical changes, which means citric acid monohydrate doesn’t just quietly decay like milk or fresh bread.
Risks of Pushing Past the Limits
Let bags go unsealed, or stock stored in a humid shed, and quality slips. Moisture draws clumps and leads to early degradation, turning crystals into sticky mush. Exposure to air or sunlight speeds this up. In food use, these shifts bring off-flavors or cloudy beverages. For cleaning, effectiveness drops off only if water or dirt gets mixed in. Citric acid rarely becomes outright harmful with age, but degraded product burns customer trust and tanks recipes.
What Matters for Safety and Quality
I check ingredient lots at home and work on a regular basis. If a pack of citric acid looks clumped, changes color, or takes on odd odors, I toss it without debate. In business, tracing stock with batch codes and rotation stops waste and keeps results consistent. Poorly tracked inventory leads to unnecessary risk or lost profits. Anyone using citric acid for food or skin contact should rely on suppliers that keep proper documentation, so if an issue ever appears, there’s a clear trail back to the source.
Better Storage, Less Waste
Extending shelf life is about basics: cool, dry, airtight storage. I’ve seen best results with moisture-proof tubs and simple silica gel packs. A kitchen cabinet or storeroom that avoids big temperature swings stops most problems. Small shops can buy just enough for a season’s demand, holding only emergency reserves. If the product never gets to those warning signs — no clumps, no color changes — and the expiration date approaches, give the oldest stock a rotation bump. Don’t wait for problems; proactive use keeps money flowing and reduces waste.
Minding the Details
Chemical safety is not guesswork. No shortcuts exist for clean handling, whether you cook at home, blend bath salts, or run a food facility. Respecting the labeled shelf life protects more than taste — it protects health, supports business reputation, and slashes needless reorders. Science has proven citric acid monohydrate’s resilience, but clear records and good habits keep that promise real for everyone who grabs a packet off the shelf.
| Names | |
| Preferred IUPAC name | 2-hydroxypropane-1,2,3-tricarboxylic acid monohydrate |
| Other names |
2-Hydroxy-1,2,3-propanetricarboxylic acid monohydrate E330 Citronensäure Monohydrat Lemon salt Citric Acid, monohydrate |
| Pronunciation | /ˈsɪtrɪk ˈæsɪd ˌmɒnəʊˈhaɪdreɪt/ |
| Preferred IUPAC name | 2-hydroxypropane-1,2,3-tricarboxylic acid monohydrate |
| Other names |
Citric acid, monohydrate 2-Hydroxy-1,2,3-propanetricarboxylic acid monohydrate Monohydrate citric acid Citrate acid monohydrate |
| Pronunciation | /ˈsɪtrɪk ˈæsɪd ˌmɒnəˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 5949-29-1 |
| Beilstein Reference | 1723201 |
| ChEBI | CHEBI:31624 |
| ChEMBL | CHEMBL1405 |
| ChemSpider | 6865 |
| DrugBank | DB04272 |
| ECHA InfoCard | 03b4be34-4866-4c6a-9784-df57a4923ade |
| EC Number | 201-069-1 |
| Gmelin Reference | 14355 |
| KEGG | C00158 |
| MeSH | D002244 |
| PubChem CID | 5949 |
| RTECS number | GE7350000 |
| UNII | 2968PHW8QP |
| UN number | UN3077 |
| CAS Number | 5949-29-1 |
| Beilstein Reference | 172422 |
| ChEBI | CHEBI:1008 |
| ChEMBL | CHEMBL1405 |
| ChemSpider | 8662427 |
| DrugBank | DB04272 |
| ECHA InfoCard | 03bba2ae-56b6-43fe-af3b-a2db8d7867be |
| EC Number | 201-069-1 |
| Gmelin Reference | 1262 |
| KEGG | C00158 |
| MeSH | D003621 |
| PubChem CID | 231 |
| RTECS number | GE7350000 |
| UNII | 2968PHW8QP |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C6H8O7·H2O |
| Molar mass | 210.14 g/mol |
| Appearance | Colorless or white crystals or crystalline powder |
| Odor | Odorless |
| Density | 1.5 g/cm³ |
| Solubility in water | 146 g/100 mL (20 °C) |
| log P | -1.8 |
| Vapor pressure | Vapor pressure: <0.1 hPa (20 °C) |
| Acidity (pKa) | 3.13 (first), 4.76 (second), 6.40 (third) |
| Basicity (pKb) | pKb: 10.32 |
| Magnetic susceptibility (χ) | -83.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.380 |
| Dipole moment | 7.45 D |
| Chemical formula | C6H8O7·H2O |
| Molar mass | 210.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Approximately 1.54 g/cm³ |
| Solubility in water | 1460 g/L (20 °C) |
| log P | -1.72 |
| Vapor pressure | Vapor pressure: <0.1 hPa (20°C) |
| Acidity (pKa) | 3.13 (1st), 4.76 (2nd), 6.40 (3rd) |
| Basicity (pKb) | 3.14 |
| Magnetic susceptibility (χ) | -8.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.333 |
| Dipole moment | 6.46 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 248.39 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1622.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2061 kJ/mol |
| Std molar entropy (S⦵298) | 242.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1540.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1986 kJ · mol⁻¹ |
| Pharmacology | |
| ATC code | A09AB16 |
| ATC code | A09AB13 |
| Hazards | |
| Main hazards | May cause respiratory irritation; causes serious eye irritation. |
| GHS labelling | **“Warning, GHS07, Exclamation mark, H319: Causes serious eye irritation, P264, P280, P305+P351+P338, P337+P313”** |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "May cause respiratory irritation. Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Autoignition temperature | 345 °C (653 °F) |
| Lethal dose or concentration | LD50 (oral, rat): 5400 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 5400 mg/kg |
| NIOSH | WA1225000 |
| REL (Recommended) | 30 mg/kg |
| IDLH (Immediate danger) | Not listed. |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. |
| GHS labelling | Warning; H319; Causes serious eye irritation |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | 345 °C (653 °F) |
| Lethal dose or concentration | LD50 (oral, rat): 5400 mg/kg |
| LD50 (median dose) | Mouse oral LD50: 5,040 mg/kg |
| NIOSH | WA7700000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 3 mg/kg bw |
| Related compounds | |
| Related compounds |
Citric acid anhydrous Trisodium citrate Monosodium citrate Disodium citrate Tartaric acid Malic acid Ascorbic acid |
| Related compounds |
Citric Acid Anhydrous Sodium Citrate Potassium Citrate Calcium Citrate Trisodium Citrate Citric Acid (anhydrous and hydrated forms) Monosodium Citrate |

