Calcium Lactate: Historical Roots to Modern Science
Tracing the History of Calcium Lactate
People have reached for sources of calcium since ancient times, but the story of calcium lactate really picked up in the late nineteenth century. Early chemists like Karl Wilhelm Scheele, who first isolated lactic acid, laid groundwork so later innovators could combine it with calcium to deliver a supplement that dissolved easily and tasted mild. As food industries grew and doctors saw the benefits of calcium supplementation, calcium lactate became a staple in pharmacy shelves and food processing plants around the world. The compound even found use before anyone knew its structure, used to treat calcium deficiency and support bone health long before marketing campaigns made its name familiar.
Product Snapshot
Calcium lactate is most often found as a white, almost odorless powder or granular solid. It dissolves fairly well in cold water but leaves most of its flavor behind, which helps it blend into beverages and fortified foods. A typical commercial product carries around 13% elemental calcium by weight, landing it squarely in the middle among calcium supplements—much better than calcium gluconate, but not quite as dense as calcium carbonate. It finds a home in sports drinks, mineral water, cheese-making, and tablets. Supplement companies use it to provide dietary calcium, especially for people who don’t do well with dairy. It never leaves chalky grit behind when mixed or swallowed, which definitely helps people stick with a supplement plan.
Physical & Chemical Properties
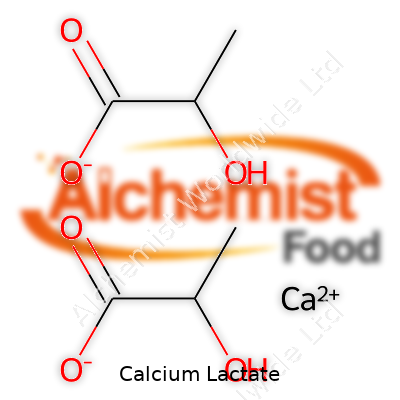
On the periodic table, calcium joins forces with lactic acid to form Ca(C3H5O3)2•xH2O, where “x” often equals five for the pentahydrate version favored in commerce. Five water molecules get locked in the structure, keeping it free-flowing and easy to handle. The substance starts to decompose above 200°C, so it works well in kitchen or industry settings that rarely reach those temperatures. In the lab, the compound gives off a slightly sweet taste, which food scientists count as a win in sensory tests. Its pH in a solution hovers a bit above neutral, which means acids don’t break it down in drinks as fast as some other salts would.
Technical Specifications & Labeling
Manufacturers hold calcium lactate to high standards, because purity and calcium content can affect everything from medical efficacy to food flavor. Food-grade calcium lactate must pass a purity test, carrying a specification like 98-101% assay by dry weight. Labels for food supplements point out gluten-free, allergen-free status, and often highlight vegetarian sources for the lactic acid. Pharmacies want certificates of analysis confirming bacteria, heavy metal, and lead content below strict limits. Regulatory approval differs by region: the USA lists it in the CFR with an E number (E327) for Europe, and China’s GB standards specify minimum calcium content and permitted excipients.
Preparation Methods
Factories typically synthesize calcium lactate in large jacketed reactors by introducing lactic acid—often produced by fermenting beet or cane sugar—with calcium carbonate or calcium hydroxide. The acid and base react, creating a solution of the salt plus water and carbon dioxide. Careful process control makes sure excess acid doesn’t make its way into the final product, so pH control and thorough mixing matter a lot in the plant. After filtering out any leftover solids, the solution gets evaporated and cooled, which causes pure calcium lactate crystals to drop out. Those crystals get dried in steam or air ovens, tested, and sometimes milled into fine powders or granules suitable for food or pharma use.
Chemical Reactions & Modifications
The reactions behind calcium lactate seem simple: lactic acid neutralizes a calcium base, and the leftover ions pair up to form the salt. Some chemists tweak the formula by partially substituting other carboxylic acids or playing with the ratio of lactic acid so they create specialized forms. Amorphous calcium lactate, for example, offers higher bioavailability in some clinical studies. Food technologists investigate ways to stabilize it further using excipients or anti-caking agents. Sometimes, companies offer anhydrous or low-water-content forms for tablets, but the pentahydrate remains the workhorse for most food and beverage uses.
Synonyms & Product Names
Calcium lactate turns up in ingredient lists under names like calcium 2-hydroxypropanoate, E327, lactate of lime, or even just plain “calcium salt of lactic acid.” Chemists also call it calcium DL-lactate, since natural lactic acid has mirror-image forms, and both D and L lactic acid find their way into commercial products. Pharmacies and supplement companies brand it with simple, direct names. Grabbing a bottle off the shelf, the packaging spells out “calcium lactate” but never loses sight of its dual heritage as both a food ingredient and a nutritional aid.
Safety & Operational Standards
Safety forms the backbone of calcium lactate production, storage, and use. Regulatory agencies around the world studied it and found it safe for intended uses, but only when made from food-grade inputs. As a powder, calcium lactate dust can irritate the throat or lungs, so processing plants use dust-control vents and require protective masks or goggles. Each batch passes a battery of microbiological and chemical tests before release, and storage happens in dry, tightly sealed containers to keep moisture from causing clumps or supporting bacterial growth. For the average person, taking calcium lactate as directed poses no real risk, but overuse—like with any calcium supplement—could bring on problems like kidney stones or gastrointestinal upset.
Application Areas
The reach of calcium lactate goes much further than its original niche. In the food world, it strengthens gels for pectin-based jams, helps tofu coagulate in soymilk, and boosts the calcium levels in juices and mineral waters. Cheese producers turn to it for improving texture—mozzarella and cheddar both benefit from the compound. Sports drink formulators include it for replenishing calcium lost through sweat without changing the flavor or mouthfeel. Some toothpaste brands use it to support tooth remineralization. Hospitals and clinics still keep it around for treating calcium deficiency when better-absorbed agents aren’t available, and researchers evaluate it as a gentle alternative for pediatric or elderly patients who don’t tolerate harsher salts. In the field of molecular gastronomy, chefs use it to react with sodium alginate and make “caviar” pearls out of fruit juice—one of the more playful applications that keep laboratory science squarely on the dinner plate.
Research & Development Efforts
Scientists and engineers haven’t slowed their work on calcium lactate. Bioavailability studies probe how particle size and hydration level change the way bodies absorb the calcium. Researchers in animal nutrition fine-tune the ratio of calcium lactate to other mineral supplements for growing livestock, looking for ways to cut costs and boost health without extra additives. In labs around the globe, synthetic biologists test microbial production routes to make cleaner lactic acid with less environmental impact. Food technologists chart new uses in plant-based cheeses and meat alternatives, betting that a neutral tasting, easily absorbed calcium source will attract younger consumers. Clinical trials still weigh the pros and cons of different calcium salts for osteoporosis prevention and dental health, and published studies track long-term outcomes for users of calcium lactate versus other common sources.
Toxicity Research
Toxicologists give calcium lactate a fairly clean bill of health. Studies over many decades confirm that a normal dose—in supplements or food—does not harm humans or animals. The body deals with the lactic acid piece easily unless someone suffers from rare metabolic disorders. Regulatory agencies set upper safe limits for daily intake to avoid the well-known dangers of excess calcium, including kidney stones or changes to heart rhythm. Animal tests required by drug and food authorities track any sign of genetic mutation, reproductive harm, or organ damage at hundreds of times the normal human dose, with almost all studies returning negative results. Long-term, well-controlled trials in humans remain the gold standard for confirming safety, but the available evidence leaves doctors comfortable recommending calcium lactate to a broad cross-section of society.
Future Prospects
Looking forward, calcium lactate has a busy road ahead. The growing interest in plant-based foods and beverages creates a bigger market for fortification, as dairy alternatives often lack enough natural calcium. Researchers focus on improving the environmental footprint of lactic acid production through fermentation technology and greener calcium sources. Process engineers work on reducing dust, energy use, and loss in the supply chain. Consumer education about the different sources and benefits of dietary calcium helps drive interest not just in supplements, but in functional foods and drinks that fold the mineral right in. Innovations in encapsulation or formulation could provide timed release or easier swallowing, which helps children and elderly patients. Product developers keep their eyes on the prize—better health for consumers of all ages, delivered in the foods and drinks people already love.
What is Calcium Lactate used for?
Beyond the Chemistry Lab
Calcium lactate pops up in more places than most people realize. It shows up on the back of food packages, inside chewable supplements, and, sometimes, inside hospital supply rooms. For me, recognizing strange ingredient names on nutrition labels turned into a hobby, and that’s where I crossed paths with calcium lactate the most. It’s an additive, a supplement, sometimes a medicine — but its story travels through kitchens, clinics, and manufacturing floors.
Building Strong Bones and Treating Deficiency
People need calcium to keep bones and teeth healthy. Calcium lactate steps in as an alternative to other calcium salts, like calcium carbonate, especially for those who struggle with absorption. Some folks can’t handle other forms due to reduced stomach acid or certain illnesses. Doctors sometimes recommend calcium lactate tablets instead, which break down more easily in the body. A cup of yogurt or a slice of fortified bread might carry this same ingredient. In my family, we watched my grandmother switch to calcium lactate supplements because her digestion grew unpredictable with age, and the difference was clear after her blood tests improved.
A Friend to the Food Industry
Cheese makers rely on calcium lactate to set curd and enhance texture. In the world of jellies and canned fruit, it helps maintain firmness. I’ve worked in kitchens that use it as a kind of backstage hand, keeping fruits crisp and helping powders break down in water-based drinks. Calcium lactate plays well with others, dissolves pretty easily, and brings a neutral taste that doesn’t mess with recipes.
Anyone who mixes supplements will spot calcium lactate in powder blends. It acts as a source of calcium in sports drinks and effervescent tablets, especially for products aimed at people looking for smoother digestion. Compared to other salts, it gives a lower dose per gram, but it’s easier on the stomach and tends to avoid constipation that sometimes follows calcium carbonate. Anyone who’s dealt with calcium supplements knows how much the body can rebel against the wrong source.
Healthcare and Beyond
Hospitals keep calcium lactate on hand in case of low blood calcium, which can mean anything from muscle cramps to heart troubles. In some poisoning cases, doctors give it to counteract the effects of magnesium sulfate. Dentists sometimes use calcium lactate as a rinse to reduce tooth sensitivity and to speed up enamel recovery after whitening procedures.
Old construction workers talk about calcium lactate in cement and plaster to aid setting and durability, while those with technical expertise in water treatment speak about it helping stabilize water. Fewer people see it outside food and medicine, but its reach stretches further than many think.
Safety and Consumer Trust
Calcium lactate stands on solid ground in terms of food safety. It received approval from the FDA as “generally recognized as safe” (GRAS). The European Food Safety Authority checked it too. Most reactions — if they occur at all — come from high doses, not from trace amounts in foods. People with kidney problems or on certain medications should be careful, but the ingredient itself isn’t what causes trouble.
Why It Deserves Attention
Nutritional gaps keep growing as more people eat processed foods. Calcium lactate gives suppliers and home cooks an easy way to support bone health and prevent deficiency without adding strong flavors or stomach trouble. Governments and health organizations recommend regular calcium intake, so this ingredient fits into many fortification strategies.
As with any supplement, smart choices matter. People need to know where their calcium comes from and why it’s added. It’s worth a closer look, not just by reading the label but by learning how these hidden helpers work.
How should Calcium Lactate be taken or dosed?
Why Calcium Lactate Matters
Calcium isn’t just something you find in dairy. It keeps bones tough, muscles moving, and nerves firing like they should. Calcium lactate steps in when other calcium salts upset the stomach or bring on constipation. It gets the job done for those needing an edge in bone strength, especially if regular calcium pills haven’t been friendly. Anyone juggling osteoporosis, muscle cramps, or calcium-deficient diets often hears about this option. Yet, throwing back a handful of tablets won’t deliver results. The body likes a steady hand, not extremes.
What Experience Teaches Us
Doctors talk about milligrams and dosages, but most folks just want to know what works in daily life. I’ve watched people try to catch up on months of poor calcium intake with one heavy dose. More problems show up this way—digestive discomfort or even kidney stones. My family sticks to split doses, usually two or three times a day, with meals. Sticking to the labeled serving gives better results than guessing or doubling up. For adults, the typical calcium intake from all sources should land around 1,000 to 1,200 milligrams daily, counting food and supplements together. Exceeding that quietly raises risks, without giving much benefit.
Food plays a big part. Calcium lactate absorbs best with a meal, not on an empty stomach. Skipping that step means the body doesn’t grab as much calcium. Mixing up diet with greens like broccoli and kale, plus dairy, lets supplements fill the gaps instead of carrying the whole load. For anyone with kidney problems or parathyroid issues, extra caution matters. Doctors sometimes run blood and urine checks before green-lighting any supplement routine. These tests can dodge trouble before it shows up.
Common Sense Around Supplementation
A little discipline goes further than guesswork. I keep a pillbox handy, set a phone alarm, and forget about it otherwise. Calcium tablets don’t taste great if chewed, so swallowing with water works best. Forgetting doses once in a while doesn’t mean big trouble, but don’t double up. Consistency wins out. Brands can differ in how they package calcium lactate, often either regular tablets or chewables. Read the label for how many milligrams of “elemental calcium” each serving gives—not just the total weight.
Adding vitamin D opens the door for better calcium use by the body. Milk, sun, or a small vitamin D capsule can work just as well. Avoiding heavy caffeine and sodium means less calcium leaves through urine. For folks on heart drugs, antibiotics, or thyroid pills, spacing out the supplement by at least two hours avoids mix-ups.
Seeking Better Results
Ignoring dosing advice rarely ends well. Chatting with a healthcare provider is more than a formality—some pre-existing health issues or drug interactions create roadblocks. Pharmacists know which brands have clean ingredient lists, and some doctors have seen enough cases to spot problems before they grow. If muscle cramps, tingling, or stomach pain rear up, don’t brush it off. That’s a cue to re-evaluate what, how, and how much gets used each day.
More folks today balance busy lives with real health concerns. Calcium lactate won’t solve every problem, but smart routines let it play its part. The real win comes from steady habits, balanced meals, and learning from the past.
Are there any side effects of Calcium Lactate?
Why People Turn to Calcium Lactate
Many of us try to keep bones strong, especially as we get older or if someone in the family had osteoporosis. Lots of doctors and nutritionists suggest adding more calcium to your diet. Calcium lactate makes its way into powders, tablets, and even some sports drinks. Grocery shoppers might spot it on food labels, too. This form absorbs easily and works for people who need extra calcium but struggle with other supplements.
Understanding the Risk of Side Effects
Some folks reach for supplements and assume “natural” means “completely safe.” That easy confidence sometimes leads to trouble. Calcium lactate, like any supplement, has possible side effects. Most people notice nothing, but a few run into stomach problems. Upset stomach, constipation, and bloating crop up, especially if someone doubles their usual dose or skips drinking water. I've witnessed this first-hand with patients who started taking higher amounts to help their bones, only to find themselves constipated. Extra fiber, more fluids, or a quick chat with the doctor usually sorts things out.
More Isn’t Always Better
Calcium supplements help, but it’s possible to get too much of a good thing. Health experts call this “calcium overload.” Going way beyond the suggested daily amount may leave you with kidney stones or trouble absorbing other minerals like iron or zinc. I remember a neighbor who popped calcium tablets like candy, thinking bones needed all the help they could get. After a few months, he landed in the ER with severe lower back pain. The culprit turned out to be a kidney stone—something his doctor linked back to long-term excess calcium intake.
Interactions Can Sneak Up
Mixing medications and supplements brings some hidden dangers. Calcium lactate can mess with the absorption of certain antibiotics or thyroid medicines. Anyone who regularly takes prescription pills needs to flag this during doctor visits. Pharmacists often catch these things, but people still need to speak up. The FDA highlights that calcium should be taken a few hours apart from some antibiotics to avoid problems with absorption.
Who Should Be Extra Careful?
Not everyone needs the same amount of calcium each day. People with kidney disease, a history of kidney stones, or parathyroid problems need to stay alert. Anyone with a medical condition or on long-term medication should check with their healthcare provider before loading up on any kind of supplement.
Ways to Stay Safe With Calcium Lactate
Read the label before opening a new bottle. Stick to recommendations from trusted health groups or doctors. If possible, look for calcium in food—leafy greens, dairy, even fortified plant milks—so you get benefits without surprises. Let your doctor know about all supplements and keep an eye open for tummy troubles, pain, or sudden health changes. Too many people skip this step, thinking supplements are outside their doctor’s concern.
Final Thoughts
The idea that more vitamins or minerals always leads to better health simply doesn’t hold up. Calcium lactate offers real benefits, especially for people who can’t get enough through food. At the same time, respecting the possible side effects and keeping doctors in the loop remains the best step for strong bones—and good health all around.
Is Calcium Lactate safe for pregnant or breastfeeding women?
What Calcium Lactate Does in the Body
Calcium lactate carries out an essential job—supplying calcium, which bones, muscles, and teeth demand daily. For pregnant women, calcium helps the baby’s bones develop without depleting the mother’s own stores. Breastfeeding mothers also hand off a steady supply of calcium to their infant through milk. Too little calcium can leave both mother and child vulnerable to bone loss, cramps, or heart rhythm issues.
Digging into Safety Concerns
Concerns surface with any supplement or additive—pregnancy hormones shift how the gut absorbs minerals, and women want food and medicine that won’t harm the baby. Calcium lactate lines up as a safe option according to several leading sources, including the US Food and Drug Administration (FDA) and European Food Safety Authority (EFSA). No major study has reported risks from typical calcium lactate levels in food or supplements used as part of a balanced diet.
Doctors have often recommended calcium supplements for women who don’t get enough through food. Compared to other calcium salts like calcium carbonate or calcium citrate, calcium lactate dissolves well, making it gentler on the stomach. Women with a history of constipation or bloating sometimes prefer it for that reason.
Potential Drawbacks
Still, no supplement is one-size-fits-all. Side effects are rare but can include mild gas or an upset stomach. Taking more than a doctor recommends can cause kidney stones or interfere with how the body absorbs iron and zinc, two other minerals pregnant women can’t afford to lose. Some people with kidney disease need to watch calcium intake, and pregnant women with health problems benefit from discussing all supplements with an obstetrician or midwife.
What Science and Guidelines Recommend
Real world studies back up the safety profile. The Institute of Medicine recommends about 1,000 mg of calcium for most pregnant and breastfeeding women, a target that includes both food and any supplements. Lactose intolerance and plant-based diets can make that target harder to hit with food alone, nudging some women toward calcium lactate tablets or powders. Dairy, leafy greens, and fortified juices remain excellent first choices, but supplements fill the gap safely for those who need it.
What to Look for in Practice
Reading ingredient lists helps avoid unwanted additives and keeps intake in a safe range. Some prenatal vitamins contain calcium lactate, but labels show exactly how much each tablet delivers. Splitting the dose through the day supports absorption, and taking calcium at a different time from iron supplements avoids interference. Doctors also test blood calcium in those with thyroid or kidney concerns, giving a safety net against too much.
Exploring Solutions and Smart Habits
Clear guidance makes all the difference. Regular conversations with healthcare professionals can fine-tune supplement choices and address any changes that come up in pregnancy or nursing. Free community programs and better food labeling would help all families get the nutrients needed to grow strong kids without extra risk. Women’s health organizations push for research including pregnant and breastfeeding populations, closing the gap where data or reassurance still feels thin.
Calcium lactate stays on the list of safe, trusted resources for women taking care of their bone health—and their children’s—right from the start.
Can Calcium Lactate interact with other medications?
Understanding Calcium Lactate
Calcium lactate pops up in everything from food additives to dietary supplements. Some folks use it to plug gaps in their calcium intake, some doctors recommend it for bone health, and sometimes it appears on ingredient lists you barely notice. It seems harmless on the surface, but combining it with certain medications could throw a wrench in your health plans.
Everyday Medications and Calcium Lactate
One routine trip to a pharmacy makes it clear how often people juggle several medications. Pain relievers, thyroid pills, blood pressure drugs, and antibiotics line the medicine cabinets of millions. Mixing supplements with these isn’t always as easy as throwing them in the same pillbox.
Calcium in any form, including calcium lactate, can mess with the way our bodies absorb other medications. It can cling to antibiotics like tetracycline and ciprofloxacin, basically forming clumps that pass through the gut without doing their job. That means infections stick around longer and that course of antibiotics doesn’t deliver the results the way it should.
People taking thyroid hormones like levothyroxine need to pay close attention, too. Calcium supplements can block thyroid medication from getting into the bloodstream, leading to fatigue and other symptoms, as if they hadn’t taken their prescription at all. Even several hours apart, enough calcium can still cause problems if your body takes its time absorbing nutrients.
Kidney, Heart, and Bone Medications
Patients working through bone issues with bisphosphonates rely on pills like alendronate or risedronate. These need a clear path into the body to be effective, and calcium can block the way. The drugs don’t reach the bones if the gut throws up a calcium roadblock.
Blood pressure medications—some types, like calcium channel blockers, already play with the body’s mineral balance. Extra calcium from supplements might tilt that balance more than you’d expect. People using digoxin or certain water pills called thiazides have seen spikes in calcium levels that cause confusion, muscle weakness, and even heart rhythm changes. Ignoring these risks comes at a cost.
Gut Health and Absorption Issues
Some medical conditions make people prone to nutrient absorption problems. Crohn’s disease, celiac disease, and chronic diarrhea mean even trace changes in the gut can make a big difference. In these cases, throwing extra calcium lactate into the mix can worsen blockages or reduce the effectiveness of prescription drugs meant to support gut or bone health.
Keeping Things Safe: Practical Tips
Checking for interactions means starting with your doctor or pharmacist—a quick conversation makes all the difference. Bringing a current list of everything you take, from herbal teas to over-the-counter pills, gives healthcare workers the info they need to spot trouble before it starts. Many pharmacists recommend leaving at least two hours between calcium supplements and other medications. Reading labels, asking questions, and not treating “natural” as “risk-free” all make a difference. No one wants to see their hard-earned efforts undone by something as preventable as timing.
For anyone who feels unsure, simple blood tests can quickly check if calcium levels are too high or too low after starting a supplement. This catches issues early and lets doctors adjust medications before symptoms get serious.
| Names | |
| Preferred IUPAC name | calcium 2-hydroxypropanoate |
| Other names |
Calcium 2-hydroxypropanoate Calcium lactate pentahydrate E327 |
| Pronunciation | /ˈkæl.si.əm ˈlæk.teɪt/ |
| Preferred IUPAC name | calcium 2-hydroxypropanoate |
| Other names |
E327 calcium salt of lactic acid calcium 2-hydroxypropanoate |
| Pronunciation | /ˈkæl.si.əm ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 814-80-2 |
| Beilstein Reference | 3919708 |
| ChEBI | CHEBI:31341 |
| ChEMBL | CHEMBL1201543 |
| ChemSpider | 5202 |
| DrugBank | DB11093 |
| ECHA InfoCard | ECHA InfoCard: 03-2119969274-33-0000 |
| EC Number | 814-800-6 |
| Gmelin Reference | Gmelin Reference: **83241** |
| KEGG | C14814 |
| MeSH | D002121 |
| PubChem CID | 3032639 |
| RTECS number | EW5425000 |
| UNII | 9UUC548I6E |
| UN number | UN1847 |
| CAS Number | 814-80-2 |
| Beilstein Reference | 1721812 |
| ChEBI | CHEBI:31341 |
| ChEMBL | CHEMBL1201563 |
| ChemSpider | 54618 |
| DrugBank | DB11093 |
| ECHA InfoCard | 11fa0e6d-091c-4a51-b0c8-1372ecf13add |
| EC Number | 814-245-4 |
| Gmelin Reference | Gmelin Reference: **81187** |
| KEGG | C01742 |
| MeSH | D002121 |
| PubChem CID | 3034409 |
| RTECS number | FF8050000 |
| UNII | 9U6U1N70BQ |
| UN number | UN1781 |
| Properties | |
| Chemical formula | C6H10CaO6 |
| Molar mass | 218.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 0.8 g/cm³ |
| Solubility in water | 6.7 g/100 mL (20 °C) |
| log P | -2.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.8 |
| Basicity (pKb) | 11.8 |
| Magnetic susceptibility (χ) | -52.0e-6 |
| Refractive index (nD) | 1.5 |
| Dipole moment | 5.983 D |
| Chemical formula | C6H10CaO6 |
| Molar mass | 218.22 g/mol |
| Appearance | White crystalline or granular powder |
| Odor | Odorless |
| Density | Dense powder, 0.7 g/cm³ |
| Solubility in water | 8.7 g/100 mL (20 °C) |
| log P | -2.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.5 |
| Basicity (pKb) | 12.6 |
| Magnetic susceptibility (χ) | -22.0e-6 cm³/mol |
| Refractive index (nD) | 1.52 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.50 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2056.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3221.8 kJ/mol |
| Std molar entropy (S⦵298) | Ca(C3H5O3)2(s) S⦵298 = 248.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1687.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3131 kJ/mol |
| Pharmacology | |
| ATC code | A12AA02 |
| ATC code | A12AA02 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS labelling: "Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | Keep out of reach of children. If medical advice is needed, have product container or label at hand. Store in a dry place. Store in a closed container. Dispose of contents/container in accordance with local/regional/national/international regulations. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | > 400°C |
| Lethal dose or concentration | LD50 (oral, rat): 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 5000 mg/kg |
| NIOSH | No NIOSH. |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 1000 mg |
| Main hazards | May cause respiratory irritation. May cause eye irritation. May cause skin irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture. |
| Precautionary statements | Keep container tightly closed. Store in a dry place. Store in a well-ventilated place. Wash hands thoroughly after handling. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 4190 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5,930 mg/kg (rat, oral) |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 800 - 1200 mg/day |
| Related compounds | |
| Related compounds |
Calcium gluconate Calcium chloride Magnesium lactate Lactic acid |
| Related compounds |
Calcium chloride Lactic acid Calcium gluconate |

