Calcium L-Aspartate: Tracing Development, Properties, and Future Uses
Historical Development
Back in the early days of dietary supplementation, companies searched for more bioavailable forms of essential minerals. Calcium, critical for bones and muscles, often showed poor absorption in its common forms like carbonate and phosphate. Researchers dug into amino acid chelation, finding that joining calcium to L-aspartic acid improved how the body used the mineral. By the late 20th century, Calcium L-Aspartate carved out a niche in Japan and the US as a supplement for those needing extra support. Academic curiosity led to clinical trials, and nutrition experts noticed that not all calcium salts worked equally in the gut. L-Aspartate stuck because it showed real potential in laboratory animal models and in adults struggling to keep their calcium levels up.
Product Overview
Calcium L-Aspartate stands out among calcium supplements for its blend of elemental calcium and L-aspartic acid. Instead of chalky texture and poor taste, this form dissolves better in water and goes down easier. It shows up as a white, nearly odorless powder, sometimes pressed into tablets or capsules for dietary supplements. The aspartate component serves as a shuttle, helping transport calcium across intestinal walls. Specialists often recommend Calcium L-Aspartate to adults who have trouble absorbing calcium from their food, or for those with higher physiological needs, such as athletes, pregnant women, or older adults at risk of bone loss.
Physical & Chemical Properties
This mineral salt appears as a fine, white crystal or powder, with a mild taste and no scent. It doesn’t clump up when exposed to humidity, and it mixes with water much more easily than calcium carbonate or phosphate. Chemically, the molecule contains two carboxylic acid groups from aspartic acid bound to a calcium ion. Its solubility in water, roughly 10 g/L at room temperature, means better dissolution and absorption in the body, supporting the assertion that it outperforms less soluble forms. The pH of a dissolved solution tends toward the acidic side, thanks to the aspartic acid backbone, which plays into its increased bioavailability.
Technical Specifications & Labeling
Manufacturers usually measure Calcium L-Aspartate's quality by checking for calcium content, purity, bulk density, particle size, moisture, and trace heavy metals. Top-grade products contain at least 13-14% elemental calcium by weight and almost always rank above 98% in purity. Strict labeling requirements, driven by US FDA and EU regulations, require clear documentation of both elemental calcium and the compound’s total weight. Labels should spell out all other excipients present in supplements, the country of origin, and any allergen information. Some regional agencies limit the amount of L-aspartic acid due to its potential neuroactivity, so those numbers appear too.
Preparation Method
Production starts with high-purity L-aspartic acid, made by microbial fermentation or enzymatic synthesis, and a soluble calcium salt like calcium chloride. Factories dissolve both compounds in water and let them react. Calcium ions snap up the carboxylate groups off aspartic acid, forming Calcium L-Aspartate and setting off a mild heat release. This solution goes through filtration to take out gases or solids, then gets concentrated, evaporated down, and dried into a powder. Batch records, temperature logs, and purity checks fill stacks of paperwork, especially in GMP-compliant plants.
Chemical Reactions & Modifications
At the molecular level, the reaction forms a chelate where the calcium binds with both oxygen atoms on aspartate. With heat or strong acids, the compound can release its calcium as a free ion and reform the starting chemicals. Chemists sometimes tweak the aspartate backbone by attaching methyl or ethyl groups, trying to fine-tune solubility or absorption. Most of these modifications remain in experimental labs since they rarely beat the parent compound in performance or safety. Blending with vitamin D or magnesium can support uptake and create synergistic health products.
Synonyms & Product Names
Alongside 'Calcium L-Aspartate,' industry catalogs mention names like 'Calcium Diamino Succinate,' 'Calcium L-Aspartic Acid,' or abbreviate it to 'Ca-L-Asp.' Supplement shelves show branded terms such as 'Aspartate Calcium' or 'Amino Acid Chelated Calcium,' confusing consumers comparing raw ingredient lists. Though names vary, precise chemical identity makes or breaks the trust in what people swallow.
Safety & Operational Standards
Regulated by food safety authorities across North America, Europe, and Asia, the compound carries strict quality controls. The US Pharmacopeia and European Pharmacopoeia spell out the limits for elemental impurities – no more than a few parts per million for lead, mercury, or arsenic. Workers handling the powder use gloves and masks, preventing inhalation or prolonged skin contact. GMP manufacturing keeps cross-contamination away, and audits check for microbial safety, identity, and consistent quality. The FDA classifies it as a Generally Recognized as Safe (GRAS) ingredient at ordinary doses, but high intakes prompt closer review because amino acid components behave differently in some rare metabolic disorders.
Application Area
Dietary supplements remain the largest market for Calcium L-Aspartate, pushed by evidence favoring more bioavailable calcium for people at risk for osteoporosis or athletes who sweat out minerals. Pharmacists recommend it when patients cannot tolerate carbonate or citrate due to digestive issues. In food technology, this additive improves nutritional labels for plant-based drinks or meal replacements, where dairy isn’t possible or desirable. Research looks at its potential in sports nutrition and even as a therapy add-on for certain neuromuscular conditions. Outside human consumption, a few animal nutrition companies test it in livestock rations where fast growth and high mineral absorption count the most.
Research & Development
Academic teams and supplement companies continue to chase new angles. Universities monitor long-term impacts of aspartate-bound minerals on bone density in post-menopausal women and on muscle cramps in athletes. Other studies compare how diets high in phytates or oxalates interact with Calcium L-Aspartate compared to other calcium salts. Medical journals document absorption rates using isotope tracing, adding weight to practical advice given by nutritionists. Some R&D teams focus on optimizing particle size for easy dissolution, while others play with microencapsulation to mask taste or combine with vitamins. A few public health projects look at fortifying staple foods in regions with endemic calcium deficiency, weighing cost against absorption advantages.
Toxicity Research
Toxicologists bombarded animal models with high doses, searching for neurotoxicity or organ damage. L-aspartic acid, as an excitatory neurotransmitter, caused some early alarm, but realistic human doses show little risk for healthy adults. Rare genetic disorders such as phenylketonuria or urea cycle defects make aspartate metabolism a problem, so doctors advise caution in those cases. Published research returns again and again to typical daily consumption levels, showing no evidence of calcium overload nor buildup of aspartic acid in most people. Digestive side effects, such as bloating or diarrhea, stay below those seen with calcium carbonate.
Future Prospects
Worldwide demand for well-absorbed, gentle-on-the-stomach calcium drives new investments. Populations age, bone loss stays a leading health worry, and mineral-fortified processed foods take up more shelf space every year. Plant-based diets also lift interest in non-dairy calcium forms, shifting the market share further into chelates like Calcium L-Aspartate. Research into gut microbiome interactions might uncover more ways this compound helps or hinders nutrient uptake. With clearer guidance from nutrition experts, better clinical evidence, and steady safety records, the supplement could shift from niche status to mainstream staple, especially if manufacturing costs fall as processes scale up. Scientists continue to debate the ideal partners – should vitamin K2 or magnesium ride with it? – hoping ripple effects in cardiovascular and cognitive health make the investment worthwhile. Industry and academia keep their collective eyes on how new data changes dietary guidelines and consumer options, pushing toward safer, more affordable ways to deliver essential minerals.
What are the health benefits of Calcium L-Aspartate?
Understanding Calcium L-Aspartate
Calcium L-Aspartate draws attention for a good reason: it’s more than just another form of dietary calcium. This compound, made from calcium and the amino acid aspartic acid, gets absorbed efficiently by the body. That means you don’t see as many of the typical issues people complain about with plain old calcium carbonate, like bloating or constipation.
Bone Health: More Than Just Hype
Strong bones matter, and calcium L-aspartate actually delivers. The skeletal system needs enough calcium, and researchers like those at the National Institutes of Health highlight that most Americans simply do not get enough through food. Chronic calcium deficiency can trigger issues like brittle bones or osteoporosis. Instead of loading up on high-dose calcium carbonate or citrate, which sometimes get excreted before doing much good, this form allows the body to take in more of what you swallow.
Unlike traditional supplements, I’ve seen people report fewer digestive complaints with calcium L-aspartate. This flexibility lets older adults, athletes, or anyone needing extra calcium get what they need without adding stress to their stomach. Doctors often prefer to recommend supplements that people can actually absorb; the aspartate form fits that bill.
Beyond Bones—Heart, Muscle, and Nerve Support
Calcium does more than patch up bones. Muscles, nerves, and the heart all count on it. If you’ve ever felt your muscles cramp after a long run or a tough day on your feet, there’s a good chance low calcium played a part. Calcium L-aspartate’s high absorption helps keep muscle contractions steady. That means less cramping—and more confidence that your body is doing what it should.
Electric signals in the heart and brain also depend on enough calcium flowing in the bloodstream. A shortage can throw off your heart rhythm, slow thought processes, or make you feel weak, especially as you age. Some published studies point to improved heart health when calcium is kept at the right level, stressing that the balance between getting enough and not overloading is critical.
Possible Help for Athletes and Active Adults
Athletes have extra demands on their nutrition. Heavy sweating causes loss of both calcium and magnesium, which plays a part in why muscle cramps seem to strike most after tough workouts. The bioavailability of this form of calcium offers a real edge for those in training, as the body can make actual use of it during recovery.
Real Food or Supplement?
Whole foods like dairy, leafy greens, almonds, or salmon will always beat out pills in terms of overall nutrition. Some folks—lactose-intolerant, vegan, or with gut issues—struggle to meet daily calcium targets this way. That’s when smart supplementation steps in. A supplement with high bioavailability, like L-aspartate, can fill the gaps.
Making the Most of Supplementation
As with all nutrients, more isn’t always better. The FDA points out that too much calcium can cause kidney stones and even interfere with the absorption of iron or zinc. Checking with a healthcare provider ensures you get enough—without overdoing it. Blood tests, a dietary log, or advice from a registered dietitian shine a light on what you really need.
Calcium L-aspartate offers a useful alternative for those needing reliable calcium support beyond what they get from food. In my own experience advising friends and family, those who switched to aspartate-based supplements noticed greater comfort and—over time—improved bone density numbers on their regular checkups. That sort of track record speaks for itself.
How is Calcium L-Aspartate different from other calcium supplements?
A Different Kind of Calcium
Calcium fills a big gap in many diets. Most people think of chalky tablets or big vitamin bottles packed with calcium carbonate or citrate. L-aspartate belongs to a group not found on every pharmacy shelf. The difference comes down to how this type works inside the body, and how much users actually absorb from their dose.
The Science Behind Absorption
Most folks take calcium expecting to build stronger bones or supplement a dairy-light diet. It’s no secret: just because a bottle says “calcium” doesn’t mean your body gets all of it. Some types pass through without much effect. A lot of what you swallow is lost for one reason or another. Calcium carbonate, for example, breaks down best in the acidic environment of a full stomach. Many antacids use this same compound, but not everyone digests it well. Calcium L-aspartate, on the other hand, couples calcium to the amino acid aspartic acid. This match-up leads to a more friendly, bioavailable form—meaning the body takes in a higher percentage with each pill.
Gentle on the Stomach
Stomach drama can push people to stop using calcium altogether. Standard calcium carbonate can cause bloating or constipation, and it generally asks for food in the stomach to help with absorption. Calcium L-aspartate lands much gentler on the gut and doesn’t depend so much on food to work. This small change can make the difference between sticking to a supplement routine and giving up.
Beyond Bones: Supporting Nerves and Muscles
Most folks look at calcium for their bones, but the story is bigger. Every heart beat, muscle twitch, and nerve signal relies on calcium. L-aspartic acid, the companion in this combo, plays its own part in energy production for your cells. Some early studies hint this unique form may bring a broader benefit for muscle performance and daily energy, aside from the bone health everyone expects. That’s a big deal for active workers, school kids, and folks aiming to age well.
Who Benefits the Most?
Not everyone needs the same kind of calcium. Those who struggle with digestion, take medications that reduce stomach acid, or have older guts naturally set themselves up for less absorption from standard pills. Calcium L-aspartate’s structure sidesteps some of these hurdles. That’s a plus for older adults and people with chronic conditions.
Naturally Occurring Advantage
Some forms of calcium use synthetic binders or require a lot of additives. Calcium L-aspartate exists naturally in foods like sugar beet molasses, and its absorption in the body reflects that origin. Compared to some rock-based forms, it feels closer to what would show up in a balanced diet. That natural link matters to folks who value clean labels and whole-food nutrition.
Areas for Growth and Caution
Despite its promise, L-aspartate isn’t as widely studied as old-school forms like carbonate or citrate. Most research sits at the preliminary stage. Doctors and nutritionists remain cautious, wanting more evidence on long-term use. Anyone with chronic kidney disease or mineral imbalances needs oversight from a healthcare provider—too much of any mineral can throw off the body.
Looking Ahead
Shoppers face a busy supplement aisle. Calcium L-aspartate gives a real alternative, especially for those not thriving on standard products. Choosing a supplement still comes down to listening to the body, reading ingredient lists, and checking in with a trusted provider. Science keeps unfolding, but every new form that supports absorption and gentler digestion counts as a win for those who need a little extra help rebuilding or maintaining their strength.
What is the recommended dosage for Calcium L-Aspartate?
Why Care About Daily Calcium?
Strong bones rely on steady calcium. That’s something my parents drilled into my siblings and me growing up, especially every time milk hit the dinner table. It’s not only about bones, though. Calcium plays a role in muscle contraction, nerve signaling, and even heart health. When food habits change, and dairy or leafy greens don’t show up enough, supplements like Calcium L-Aspartate become practical options. Missing enough calcium over months or years genuinely raises the risk of brittle bones or muscle cramps—stuff that tends to catch people off guard as birthdays add up.
What Doctors Recommend
Experts, including the National Institutes of Health, usually set calcium requirements around 1,000 milligrams daily for adults. That need climbs to 1,200 milligrams for women over 50 and men past 70. Calcium L-Aspartate is one specific compound that combines calcium with aspartic acid, a type of amino acid. Unlike some calcium salts, this version often gets noticed for its decent absorption and gentler impact on digestion. Still, the overall calcium amount coming from the supplement is what truly matters. Most calcium L-aspartate capsules contain about 100 to 200 milligrams of elemental calcium—the “usable” part—per serving. That means just swallowing one pill likely won’t fill the daily need, especially for someone skipping dairy and greens. Checking labels and matching up the total with your actual intake from food makes a difference. Overshooting calcium for long stretches brings risks, too. Too much, especially past 2,000 milligrams a day, can hurt the kidneys and might even help kidney stones form. So more is not always better.
Why Individual Needs Vary
Doctors look at meals, age, medications, and sometimes even blood work before giving thumbs-up to a supplement plan. Heart or kidney conditions sometimes call for avoiding extra calcium unless there’s a proven need. People who take thyroid medicine, diuretics, or certain antibiotics can find calcium interfering with how much their body absorbs these drugs. My aunt, for example, had to plan her calcium hours apart from her morning thyroid pill. Without checking with a healthcare provider, it’s easy to guess wrong and either miss out or tip the scale toward too much.
Solutions for Common Roadblocks
Getting enough calcium from food is tough for folks with lactose intolerance or certain allergies. Plant milks (fortified with calcium) and greens like collard or bok choy help. For those who turn to supplements, setting reminders and taking smaller divided doses with meals helps the body soak up more. Splitting up a 1,000 mg daily dose into two or three servings works best since the body can’t use a huge chunk of calcium in one go.
Doctors and dietitians remain the go-to guides for personalizing the plan, especially since blood calcium levels and overall nutrition play a role in health over decades. They also keep tabs on which products actually list how much calcium is inside. With so many supplement jars crowding shelves, trusting a product that’s been tested or recommended by a reliable health source protects against under-filling or contamination—problems the FDA sometimes finds during spot checks.
The Takeaway
Calcium L-Aspartate delivers on the promise of helping folks meet their daily needs, but it’s not a one-size-fits-all solution. Reading nutrition labels carefully, staying under the upper limit, and working with a care provider takes the guesswork out of dosing. Daily calcium decisions shape bone strength, muscle performance, and even heart health, and nobody gets a free pass just because they reach for a supplement bottle instead of a glass of milk.
Are there any side effects or precautions with Calcium L-Aspartate?
Getting Real About Calcium Supplements
Calcium L-Aspartate often lands in the hands of folks looking to strengthen their bones or correct a dietary gap. This salt form promises better absorption than basic calcium carbonate, and some fitness circles swear by it. Still, as with so many things, the story doesn’t end with marketing claims or slick packaging. Calcium supplements have side effects, even those on the “gentler” end of the spectrum.
Digestive Woes: The Common Complaint
It’s tough to avoid stomach discomfort with many calcium supplements. Calcium L-Aspartate does a little better here, but it can still leave people feeling bloated, gassy, or stuck with constipation. Folks who take high doses, or swallow their supplements all at once without enough water, usually feel it worse. Over the years, I’ve noticed friends and relatives groan about being irregular after a few weeks on calcium, especially without much fiber in their diet.
Balancing Blood Calcium Levels
Blood tests tell a clear story—a steady rise in blood calcium, or hypercalcemia, spells trouble. Anyone pounding the pills without guidance, or mixing calcium supplements with high-calcium foods, opens the door to kidney stones. Most doctors agree: keep total intake below 2,000–2,500 mg daily for adults. The U.S. National Institutes of Health drives this number home. Overdoing it can mean more than gritty kidney stones; it can put the heart and nerves at risk too.
Interactions With Other Medications
Many people forget that supplements can block or boost how other medicines work. Taking Calcium L-Aspartate with thyroid drugs, certain antibiotics (like tetracyclines or quinolones), or blood pressure meds can haul down the medicine’s effectiveness or slow down absorption. It pays off to ask a pharmacist before starting. Just the other day, a friend taking thyroid medicine lost his energy—turned out his morning calcium pills were getting in the way.
Special Populations: Who Should Watch Closer
Children, seniors, and pregnant women have different calcium needs, and their bodies can react in unexpected ways. Chronic kidney disease ramps up the risk of calcium build-up, since damaged kidneys can’t clear excess minerals. A neighbor with moderate kidney disease told me her doctor kept her calcium supplements locked away for this reason. She now leans heavily on food sources, steering clear of unnecessary pills.
Safety Starts With Simple Habits
The best move starts at the dinner table. Aiming for calcium-rich foods—dairy, leafy greens, nuts—keeps things natural and usually safe. If a supplement feels necessary, smaller doses spread throughout the day work better and cause fewer problems. Chasing that big, single-dose fix does more harm than good.
Always check with a clinician before adding any supplement, especially with pre-existing conditions or regular medication use. Dietary decisions that help balance minerals usually go a long way toward avoiding the most troubling side effects. Supplements fill gaps, but they don’t patch every leak.
Looking Ahead
A bit of homework, honest questions for the doctor, and a strong focus on diet can turn a potentially tricky supplement into one piece of a much bigger health puzzle.
Is Calcium L-Aspartate suitable for vegans and vegetarians?
Understanding the Source
Calcium L-Aspartate often pops up on supplement shelves, promising to boost calcium intake without the chalkiness of some old-school tablets. Looking at the label, the story seems simple: calcium bound to an amino acid called aspartic acid. Many shoppers, especially those who avoid animal products, want to know what’s really behind those chemical-sounding phrases.
Tracing Ingredients to Their Roots
Aspartic acid is one of those building blocks present in most living things, but industry sourcing matters. Most aspartic acid in supplements comes from fermented plant sources like sugar beets or molasses. Manufacturers use bacteria to help break down plant starch into amino acids. That’s how the powder ends up in the supplement jars. On the calcium side, it usually comes from mineral ores, not animal bones or shells.
I remember reaching out to several reputable companies and asking about their processes. The ones that take pride in serving vegan customers often send over third-party documentation, showing plant-based fermentation or confirming no animal derivatives touched the process. This transparency gives plant-based eaters peace of mind. Still, not every supplement company follows this path. Some rarely publish the origins of their raw materials, which leaves shoppers guessing.
The Small Print: Binders, Fillers, and Capsules
Vegan and vegetarian shoppers have learned to look past the front label. Capsules sometimes use gelatin, which is made from animal collagen. Fillers like magnesium stearate may be plant-sourced or animal-derived, depending on the supplier. I’ve had to switch brands before, just because the fine print revealed a sneaky animal-based ingredient. It’s not just about the main compound—the whole recipe matters.
Manufacturers that care about transparency put “vegan” or “vegetarian” verification right on the label. The best ones back it up with certifications from third-party groups such as the Vegan Society or similar organizations. These seals mean more than marketing slogans—they reassure shoppers that both ethics and health concerns line up with the product.
Reasons to Seek Out Plant-Based Supplements
For vegans and vegetarians, choosing animal-free sources goes beyond diet. Some avoid animal products for environmental reasons, aiming to cut down the resource-heavy process of raising livestock. Others feel strongly about minimizing harm. Even in non-food supplements, these values persist.
A 2022 report from the Good Food Institute points to growing demand for clear vegan and vegetarian labeling, linking it to stronger consumer trust. People don’t like to gamble with their food and supplement choices—they want clarity and control.
How Companies Can Do Better
Companies could earn trust by fully disclosing the origins of every ingredient. This means sharing not just the source, but also the refining or fermentation process. Answering emails quickly and clearly goes a long way, too. When shoppers ask questions, they want straight answers.
Regulators could also step up. Rules that require firms to label “vegan” or “vegetarian” only after proving ingredient origin and cross-contamination standards would help everyone. More transparency, fewer surprises.
Until industry standards improve, it pays to read ingredient lists with care. Look for trusted certifications, ask questions, and pick brands that value honest conversations. The more companies see that people care about these details, the more likely clean, animal-free products will become the norm.
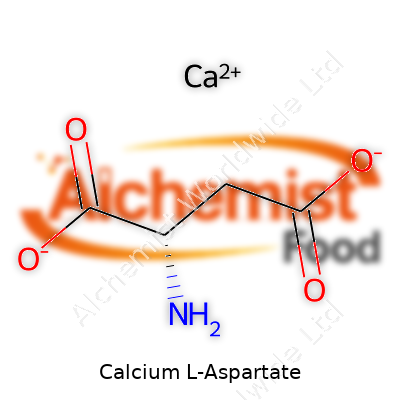
| Names | |
| Preferred IUPAC name | Calcium (2S)-2-aminobutanedioate |
| Other names |
Aspartic acid calcium salt Calcium di-L-aspartate Calcium L-2-aminosuccinate |
| Pronunciation | /ˈkæl.si.əm ɛl əˈspɑːr.teɪt/ |
| Preferred IUPAC name | Calcium bis[(2S)-2-aminobutanedioate] |
| Other names |
Aspartic acid, calcium salt Calcium di-L-aspartate Calcium L-2-aminobutanedioate |
| Pronunciation | /ˈkæl.si.əm ɛl æsˈpɑːr.teɪt/ |
| Identifiers | |
| CAS Number | 38968-95-3 |
| Beilstein Reference | 3921683 |
| ChEBI | CHEBI:31376 |
| ChEMBL | CHEMBL1201185 |
| ChemSpider | 109771 |
| DrugBank | DB11127 |
| ECHA InfoCard | 03c4a8e4-7141-4c7f-861c-87d35b5d793d |
| EC Number | E386 |
| Gmelin Reference | 189580 |
| KEGG | C14524 |
| MeSH | D017942 |
| PubChem CID | 16213504 |
| RTECS number | MR8200000 |
| UNII | 19R8B1V773 |
| UN number | UN number: "UN2811 |
| CompTox Dashboard (EPA) | DTXSID2045276 |
| CAS Number | ['19433-82-0'] |
| Beilstein Reference | 172736 |
| ChEBI | CHEBI:31375 |
| ChEMBL | CHEMBL1201782 |
| ChemSpider | 22264 |
| DrugBank | DB11126 |
| ECHA InfoCard | 03e2e3a7-3967-47c7-85e8-b097ed68bc33 |
| EC Number | 212-014-8 |
| Gmelin Reference | 321286 |
| KEGG | C13925 |
| MeSH | D013683 |
| PubChem CID | 16211083 |
| RTECS number | RR0350000 |
| UNII | 9J37B0FL1A |
| UN number | UN number not assigned |
| CompTox Dashboard (EPA) | DTXSID7020182 |
| Properties | |
| Chemical formula | Ca(C4H5NO4)2 |
| Molar mass | 324.29 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Density: 0.5 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -4.22 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.9 |
| Basicity (pKb) | 8.64 |
| Refractive index (nD) | 1.62 |
| Dipole moment | 2.09 D |
| Chemical formula | Ca(C4H5NO4)2 |
| Molar mass | 324.29 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Density: 0.9 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -2.78 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.3 |
| Basicity (pKb) | 9.64 |
| Refractive index (nD) | 1.62 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 259.6 J·mol⁻¹·K⁻¹ |
| Std molar entropy (S⦵298) | 274.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1860 kJ/mol |
| Pharmacology | |
| ATC code | A12AA13 |
| ATC code | A12AA13 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. In case of contact, rinse immediately with plenty of water and seek medical advice. |
| NFPA 704 (fire diamond) | 1-0-0 |
| LD50 (median dose) | LD50 (median dose): 16,600 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | Recommended (REL): 6 mg/m³ |
| IDLH (Immediate danger) | Not listed |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. May cause skin irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07 |
| Signal word | No signal word |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P270, P272, P280, P302+P352, P305+P351+P338, P362+P364, P501 |
| Lethal dose or concentration | LD₅₀ (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 16,600 mg/kg |
| NIOSH | Not Listed |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 3 g |
| Related compounds | |
| Related compounds |
Calcium ascorbate Calcium citrate Calcium gluconate Calcium malate Calcium carbonate Calcium lactate Magnesium aspartate Potassium aspartate |
| Related compounds |
Calcium malate Calcium citrate Calcium gluconate Magnesium aspartate L-Aspartic acid |

