Calcium Iodate: A Closer Look from Ground Level
Historical Development
Calcium iodate didn’t just pop up in the chemical world yesterday. Mining for iodine-rich caliche ore in Chile over a century ago gave the first clues about iodine compounds living in the real world, not just the lab. Chemists isolated calcium iodate while scraping through mineral samples and soon figured out it could head into industries beyond geology. Its ties to the food supply chain started early, especially once goiter linked to iodine deficiency pushed folks to look for reliable additives, setting the stage for a legacy that keeps the salt industry humming. This compound’s early use as a dough conditioner and nutritional supplement marked a simple but key win in public health battles, especially in places where diets naturally miss out on iodine.
Product Overview
Calcium iodate stands out in a lineup of chemical ingredients for its job as a source of iodine that holds up under heat and storage. Factories churn it out as a white crystalline powder that finds a home in food premixes, animal supplements, and sometimes disinfectants. The way it sits between a trace nutrient and a feed stabilizer gives it practical importance, not just on farms but also in bakeries and food-processing plants, where reliable dosing matters every day. A lot of folks in agriculture and the food trades lean on its stability, especially in spots far from the ocean. The fact that calcium iodate keeps holding its iodine without fussing over shelf time means fewer worries about cost or waste.
Physical & Chemical Properties
At a glance, calcium iodate shows up as a free-flowing white powder, almost tasteless and without a strong smell. As a compound, its chemical formula is Ca(IO3)2·H2O, and it doesn’t dissolve well in cold water—something that shapes how companies use and handle it. It melts and breaks down at higher temperatures, but stays pretty steady at room conditions. Unlike potassium iodate, this salt stands up to rough treatment without turning into free iodine, which would just gas off and vanish. Its oxidizing nature makes it more than a bystander in chemical processes—but proper storage and care prevent unwanted surprises.
Technical Specifications & Labeling
Manufacturers keep up strict technical specs. Purity sits above 99%, and heavy metal contaminants stay far below regulatory thresholds. Moisture content gets checked because clumping threatens even mixing in industrial feed or flour, and the right particle size prevents dust clouds in mills or feed plants. Bags and drums carry clear batch numbers, warnings about oxidizers, and proper storage guides. Labels shout out compliant certifications—USP, FCC, or feed-grade—so buyers can match products to regulations and avoid inspection headaches. Regulatory harmonization across food, feed, and pharma sectors matters most where finished goods cross borders; so clear paperwork makes inspections less of a gamble.
Preparation Method
Most plants churn out calcium iodate by reacting calcium chloride or calcium hydroxide with sodium iodate or potassium iodate. In my experience around industrial setups, the recipe has to strike a careful balance: avoid waste, but land on fine, high-purity crystals. Factories minimize side reactions and run the process batchwise or in continuous flow to keep output steady. Washing and drying steps keep non-iodate minerals and trace byproducts out of the final product, since off-white powders quickly raise red flags in QA labs. Handling the parent iodate salts safely also calls for good air control, and the operators always wear dust masks, not risking their lungs.
Chemical Reactions & Modifications
Calcium iodate’s main reactivity comes as an oxidizer that releases iodine under heat or acid. Drop it into hydrochloric acid, and it liberates iodine in a flash—a reaction that has earned it some use in analytical chemistry or disinfectant production. Reducing environments break it down, so folks handling it with sulfur compounds or organic matter keep an eye out for unexpected heat. Under industrial conditions, manufacturers might tweak moisture levels or use anti-caking agents, but most try to deliver the compound as simply as possible to avoid compliance headaches.
Synonyms & Product Names
On packing slips and invoices, this chemical goes by more than one name: calcium dioxidoiodate, lautarite (in the mining trade), iodic acid calcium salt, and sometimes even just “iodate” in shorthand. European feed and food regulations often stick with “calcium iodate” or the E-number E917 on ingredient lists. In Asia and Latin America, local product codes or mineral names tie it back to native supply chains. Brand names sometimes build marketing around claims of ultra-low contaminants or “microencapsulated” forms aimed at specialty food blends or pharma applications—though the chemistry stays fundamentally the same across products.
Safety & Operational Standards
Every factory or warehouse handling calcium iodate faces straightforward but critical safety checks. It’s not a substance you want in your eyes or lungs; dust control, gloves, and eyewash stations come standard in any real-world setup. Its oxidizing power means no storing near flammable goods, acids, or strong reducers, and spill response plans always focus on dry sweeping, not hosing down, to avoid acid reactions. As a trace additive in feed and food, end users depend on the upstream supply chain to test—batch after batch—for heavy metals, dioxins, and radioisotopes. Regular audits and third-party traceability save a lot of headaches if a shipment ever sets off a recall or triggers a compliance audit.
Application Area
In animal feed, calcium iodate’s value boils down to delivering enough iodine for healthy growth, avoiding deficiency diseases like goiter or thyroid imbalance. That’s as true on midwestern American pig farms as it is in South African poultry houses. In baked goods, adding just the right pinch acts as a flour treatment agent, letting commercial bread makers skip over collapsed loaves and stick with consistent texture. Some water purification plants use it for slow-release iodine dosing— a trick that can help during emergencies or in isolated towns. Chemical labs occasionally turn to its oxidation power, especially in analytical tests needing reliable, shelf-stable iodine donors.
Research & Development
A lot of new work in the R&D world looks at how to make calcium iodate delivery even more reliable across animal and human nutrition. Innovative encapsulation makes its iodine content hold out longer in high-moisture feed, and food scientists look for combinations that cut dosing errors in automated bakery lines. Environmental chemists care about waste management: cleaning up iodate runoff to avoid groundwater contamination gets its share of funding—especially with tougher global rules around chemical effluent.
Toxicity Research
Extensive animal and cell studies back a clear safety window for this additive; at normal feed rates, risks look remote. Go much higher, and toxicity shows up through oxidative stress, and in extreme cases, symptoms like nausea, vomiting, or thyroid suppression may hit exposed workers or livestock. Chronic overexposure threatens growth and metabolism in animals—a key reason for hard federal dosing caps in both the US and EU. Test labs measure iodine residues in eggs, milk, and meat, making sure a safe food supply stays that way. Researchers track possible pollutants in manufacturing waste, since careless discharge could add unwanted iodine buildup to local ecosystems.
Future Prospects
New research aims to fine-tune calcium iodate’s balance between shelf life and environmental impact—less waste, tighter dosing controls, and lower energy use in plants all get attention. AI-powered batch quality analysis might catch faulty lots before shipping, easing worries about supply chain slips. Down the line, greener production routes from biotech pathways—engineered bacteria or enzyme catalysts—could ditch some waste-heavy chemical steps. The search for replacement antioxidants or nutraceutical blends that don't push environmental limits will likely keep calcium iodate’s role under review as food and feed regulations change.
What is Calcium Iodate used for?
Calcium Iodate and Public Health
Calcium iodate usually doesn't make headlines, but it hides in plain sight, quietly shaping public health. I first learned about it while reading about global efforts to tackle iodine deficiency disorders. Simple foods often lack iodine, leading to deep health consequences. Regions that don’t get enough natural iodine in their soil often see higher rates of thyroid problems. Here is where calcium iodate steps in as an unsung hero. It acts as a source of iodine when added to salt. Many governments, aiming to prevent goiter and mental development issues in children, choose calcium iodate as their go-to additive for iodizing salt, especially in humid countries where potassium iodate might break down too soon.
Role in Animal Nutrition
Growing up in a farming region, I often saw how animal health links back to trace minerals. Dairy cows or poultry lacking in key nutrients don’t produce as expected. Calcium iodate gets added to animal feed to address these gaps. A tiny amount keeps animals healthier and keeps their growth on track. Farm veterinarians stressed how thyroid troubles or low productivity often connect to missing iodine. Many feed producers switched to calcium iodate because it stays stable in feed, even when stored for months. Researchers keep checking its safety and effectiveness. Studies from international agencies like the European Food Safety Authority back up its use in proper amounts.
Food Fortification Beyond Salt
Some countries push for iodine fortification in foods beyond table salt—think bread, dairy, or even infant formulas. In these cases, calcium iodate often wins out, since it's less sensitive to humidity and temperature. Bread makers, in particular, prefer it because it keeps its potency through baking and storage. This matters a lot in areas where refrigeration isn’t always an option. Looking at public health outcomes, areas that switched to iodized foods showed big drops in iodine deficiency. It’s a small change in how food gets made, but it leaves a mark on national health.
Use in Disinfectants and Chemical Manufacturing
You might not expect it, but outside nutrition, calcium iodate sometimes gets used to sanitize or disinfect. A few research papers talk about its mild oxidizing feature, lending a hand in water treatment for selective purposes. The chemical sector sometimes taps into calcium iodate for specific manufacturing processes, especially where a slow-releasing oxidant comes in handy. From personal experience in university chemistry labs, the stuff is handled with respect for safety protocols. Good training and ventilation help avoid issues.
Potential Issues and Solutions
Calcium iodate isn’t magic, though. Like many food additives, using too much or not enough can lead to trouble. Overuse of any iodine source runs the risk of throwing off natural thyroid balance, so clear dosage rules matter. Regular monitoring at salt plants and feed mills helps prevent mistakes. The global supply chain also faces pressure to keep up with demand. Some producers raise concerns about the price swings that hit when supply tightens. One solution I’ve seen is more countries investing in local production. Testing equipment is getting cheaper too, letting smaller producers check iodine levels right at the source. Grassroots education—teaching communities about why iodine matters—also makes a big difference. People who understand why additives like calcium iodate end up in their food and feed tend to support these steps.
Public health bodies and local producers working together keep this process on track, using research to adjust as new data rolls in. Like so many important things, calcium iodate’s impact shows up not in flashy headlines, but in generations of kids growing up without the fog of deficiency.
Is Calcium Iodate safe for human consumption?
Understanding What Goes Into Food Additives
Oversight in food production often draws on decades of research, but not every name feels familiar at the dinner table. Calcium iodate shows up most often as a source of iodine in salt and animal feed, sparking understandable questions about safety. Many folks pay close attention to what ends up in their meals, scanning ingredient lists, and looking out for additives they don’t recognize.
Iodine: An Essential Piece of Nutrition
The human body needs iodine for thyroid function. Doctors have been warning about iodine deficiency for generations, tracing it to issues like goiter or complications in pregnancy. In my own family, stories of early 20th-century “goiter belts” came up often — stretches of the Midwest where soils ran low on iodine. That history drove regulators to add iodine to table salt through compounds such as potassium iodide or, in some places, calcium iodate. The World Health Organization and major food safety agencies agree: not getting enough iodine puts public health at risk.
What Science Says About Calcium Iodate’s Safety
Calcium iodate undergoes safety reviews much like any other food additive. It’s approved by the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), not only for salt, but sometimes as a dough conditioner in bread production. Toxicology studies looking at realistic consumption levels find no meaningful concerns. The European authorities reviewed data from animal trials and human observation, setting intake limits that land miles below anything an average person would hit by accident.
Given its low solubility compared to potassium iodide, calcium iodate often finds uses where stability is a priority. Table salt fortified with this compound keeps its iodine rock-solid even in hot, humid climates. The amount added to food works out in micrograms per serving, aligning closely with the recommended dietary allowance.
Why Chemical Names Worry People
Food labels can cause more concern than comfort, especially when words carry a whiff of the chemistry lab. Real-world experience tells me most families respond to unknowns with skepticism — until they meet a health provider or nutritionist ready to unpack the science. The complicated name alone shouldn’t steer us clear. Look at sodium chloride: better known as plain old salt. Many chemical-sounding names hide nothing more alarming than the mineral salts nature herself crafted.
Lack of Evidence for Harm – Except in Overdose
The line between “enough” and “too much” in nutrition matters. Iodine, like iron and vitamin D, helps when you need it and harms if you go hog wild. Cases of excess iodine crop up mostly where supplements stack with fortified foods. Yet, the thresholds set by regulators stick well within safe boundaries. For most consumers, actual cases of toxicity from iodized salt or food additives stay out of medical records.
Building Trust: What Can Help?
Open, accessible dialogue is key. Food producers and regulators need to keep sharing clear facts and keep research in the public view. Public trust grows slower than suspicion, especially when headlines lean toward the alarming. I find that hands-on science education at every level, from schools to senior centers, helps bridge the gap. Sources of worry often shrink in the face of a plain answer and a little patient explanation.
The safest foundation comes from regular oversight, ongoing research, and a commitment to asking — and answering — tough questions. If concerns remain, talking with a registered dietitian provides grounded and personal insight.
What is the chemical formula of Calcium Iodate?
Walking Through the Chemistry Behind Calcium Iodate
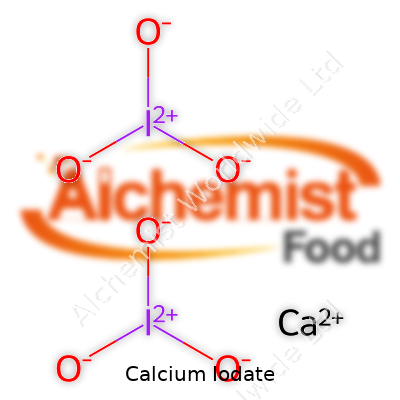
When someone asks about the chemical formula of calcium iodate, they’re diving straight into one of those topics that blends everyday chemistry with applications that touch food, farming, and even medicine. The chemical formula for calcium iodate is Ca(IO3)2. Looking at those numbers and letters might seem intimidating, but the story around them reveals why this compound matters far beyond the science class.
Why Calcium Iodate Matters
Calcium iodate often pops up where people think about nutrition and public health. Many folks have heard about iodine deficiencies, a problem that leads to thyroid issues and developmental delays. Adding iodine to table salt—iodized salt—was one attempt to tackle this. Growing up in a rural area, I saw how regional soils could miss out on these trace elements, which slowly creeps into local food supplies. Farmers and agricultural experts often turn to compounds like calcium iodate as feed additives. It gives livestock a reliable source of iodine, which in turn helps protect the health of people and animals relying on those farms.
This compound doesn’t just stop at the farm gate. Healthcare settings sometimes use calcium iodate as a disinfectant. People working with aging infrastructure, especially water systems, also know it can help reduce microbial contamination where clean water is critical. That practical approach takes the chemical formula far away from being just a textbook answer. It shows up in the daily grind of keeping food and water safe.
How the Chemistry Holds Together
Looking at Ca(IO3)2, the elements in play tell their own story. Calcium provides two positive charges (Ca2+), each iodate group balances with a single negative charge (IO3-). Put two of those iodate ions on each calcium atom, and you get a neutral molecule. This detail isn’t trivia for chemists only. Farmers and food processors depend on consistent chemistry to deliver proper doses and measurements. The right balance ensures animals and crops get the nutrients they need, but doesn’t tip over into unwanted accumulation or risk.
Production and Sustainable Use
Producing calcium iodate involves careful blending of iodine-containing compounds with calcium salts. Quality matters here. Too little purity in any step can ruin entire batches meant for feed or food use. Regulatory checks help make sure people get a safe supplement, not a potentially harmful additive. As food security conversations grow louder, reliable minerals like calcium iodate step up beyond flashy new trends. Reliable fortification hasn’t lost its importance—even as global diets and crop practices keep changing.
Looking Toward Solutions
Ensuring access to trace minerals remains one of those ongoing efforts that doesn’t always grab headlines. Governments, agricultural cooperatives, and the food industry could step up to improve public awareness about iodine and the ripple effects of deficiencies. More scientific outreach would help connect dots between raw chemical knowledge and its life-improving results. People who shape policies have a chance to support targeted fortification in at-risk areas, and continued investment in safe manufacturing practices keeps adulteration disasters at bay.
Calcium iodate isn’t the only answer to iodine deficiency, but its reliable chemistry and versatility power its continued use. The formula Ca(IO3)2 stands as more than memorized data—it supports everyday nutrition and health from the ground up.
How should Calcium Iodate be stored?
Getting Practical About Chemical Storage
Anyone who’s handled chemicals for a living knows storage isn't about ticking regulatory boxes. It's about real-world safety. Calcium iodate brings its own set of expectations to the table. As an oxidizing powder, it deserves respect: misuse means risking property and health. I’ve seen storage rooms that cut corners—open bags in humid areas, containers on corroded shelving. Those choices invite trouble.
Keep It Dry, Keep It Cool
Humidity shouldn’t get near calcium iodate. Left out in damp air, it can clump, degrade, and lose its purpose. Store the powder in a climate-controlled space. I’ve had peace of mind keeping similar chemicals far away from pipes, windows, or other moisture sources. A frost-free, low-humidity storeroom helps. Warehouse staff sometimes forget that water can sneak in through tiny roof leaks, so regular checks go a long way.
Heat changes everything. Excess warmth encourages reactions, even in so-called “stable” powders. Calcium iodate should rest below 25°C. Anything higher creates a risk—especially during summer’s hottest days. Avoid direct sunlight and don’t store chemicals near machinery that runs hot. In my work, even a simple fan improves airflow, lessening the risk of hot spots in storage spaces.
Why Sealed Containers Make a Difference
No matter how “safe” it seems, leaving calcium iodate out makes little sense. Closed containers stop airborne contamination and keep powder from attracting water or picking up odors. Good container choices include HDPE drums, thick-walled glass jars, or sturdy plastic tubs with tight lids. Metal jars won’t work, thanks to corrosion risk if any moisture forms inside.
Every workplace I’ve seen with a solid chemical program marks their containers clearly. Labels fade and smudge, so check them regularly. No one should ever open a mystery container. My advice: mark dates, batch numbers, and hazard details in plain language. Training new staff by walking them through the system prevents accidents caused by guessing what’s inside.
Segregate From Fuels, Organics, and Incompatibles
Oxidizers like calcium iodate develop dangerous tempers around flammable materials—think sawdust, oils, cardboard boxes, or common cleaning solvents. I learned fast to never tuck it next to paint cans or any organic product. A shelf lined with metal trays or plastic bins set apart for oxidizers keeps things simple and reduces mistakes. This reduces risk, especially in shared storage where people come and go with different priorities.
Cleanliness really matters. Spilled residues become accident magnets, so sweeping up powders and regularly washing sintered surfaces pays off. I recommend concrete or tiled floors that stand up to cleaning, not old wood or carpet where spills vanish out of sight. Having absorbent materials on hand helps. I’ve never regretted over-planning for spills.
Trained Hands Make the Difference
Safety policies mean nothing unless the folks doing the work understand why steps matter. Training brings out questions—why not store calcium iodate in the open? What happens if it gets wet? Sharing real incidents makes rules stick. I’ve seen labs cut risks overnight after hearing about a minor spill or a scary near-miss. Safety sheets sit on a shelf for emergencies, but good habits form at eye-level each day.
In the end, calcium iodate asks for common sense—dry, cool, separate storage in sealed and labeled containers. Layer in regular checks, teamwork, and a willingness to learn from mistakes, and you build a safer workplace where chemistry works for you, not against you.
Is Calcium Iodate the same as potassium iodate or sodium iodate?
What’s the Difference Between These Iodates?
Iodine supplements rarely make headlines, but these three names keep popping up: calcium iodate, potassium iodate, and sodium iodate. They might sound like nearly the same thing, but a closer look tells a more interesting story. Each of these compounds adds iodine to the diet, but their chemical makeup gives them unique strengths and weaknesses, often overlooked in quick online searches.
Trust in Iodine Compounds: Past, Present, and Health Impacts
Iodine comes across as something from high school chemistry, though it carries real-life weight, especially in public health. In places where thyroid problems run high, missing out on dietary iodine can mean groggy thinking and stunted growth in children. It’s not just an abstract threat, either. Cases of visible goiter and cognitive delays in remote communities prove what happens when people don’t get enough.
As a kid, I remember stacks of salt at the grocery store, some stamped “iodized,” some not. Decades back, lack of iodine in the soil forced families to deal with thyroid issues. Iodized salt stepped in as an easy fix. Governments searched for compounds that mixed easily, lasted on shelves, and didn’t taste strange. Sodium iodate and potassium iodate turned into favorites because they dissolve quickly and release iodine predictably.
Calcium Iodate: Solubility and Applications Matter
Calcium iodate takes a bit of a detour from sodium and potassium iodate. It’s usually less water-soluble, so it doesn’t dissolve in the stomach quite the same way. Sometimes, this property lets calcium iodate get chosen for fortifying animal feed or for use in baking, where slow-release can help. Potassium iodate pops up as a food additive or in emergency tablets for nuclear incidents, a fast-acting iodine source that’s easy to store. Sodium iodate works almost the same way, though it tends to find niche uses for water purification or in laboratory settings.
Manufacturers pick between them based on how fast the iodine needs to get into the body, how products are stored, and which elements won’t react with other ingredients. For example, calcium iodate holds up well under harsh handling, but potassium iodate delivers iodine more quickly when swallowed.
Safety Isn’t a Minor Detail
Supplements and food additives come with strict standards for a reason. Taking too much iodine can bring its own problems—thyroid inflammation, irregular heartbeat, and more. Both potassium iodate and calcium iodate have been studied for toxicity and are allowed in food under controlled conditions, though dosing matters. Rotating between these compounds without paying attention can spell real trouble. The Food and Agriculture Organization (FAO) and the World Health Organization (WHO) have set guidelines for acceptable use, including amounts safe for daily consumption.
Iodine isn’t just for the label, it’s for real nutrition. Using a hands-off attitude for picking the right kind can leave populations dealing with unexpected side effects. Good regulations rest on precision: testing each batch, tracking how much ends up in finished products, and offering education on proper use for industries and farmers.
Better Choices for Health and Industry
Solving iodine deficiency usually means a partnership between science, industry, and policy. Fortification projects succeed or fail based on the right blend of chemistry, safety, and honesty about benefits and risks. Switching all bakery or livestock supplements to calcium iodate just because it sounds new doesn’t work. The choice depends on how iodine stays stable, how quick it enters the human or animal system, and how trustworthy each supplier is about testing for purity.
Investing in better lab testing, ongoing education, and routine oversight raises the bar. In my experience, nutrition programs get results when all three compounds get used thoughtfully, based on real research and open communication between health officials and producers. For consumers and professionals, it pays to look past the names and pay attention to how these simple differences in chemistry can mean better—or worse—health for whole communities.
| Names | |
| Preferred IUPAC name | Calcium diiodate |
| Other names |
Calcium diiodate Lime iodate Iodic acid, calcium salt Calcium iodate(V) Iodate de calcium |
| Pronunciation | /ˈkæl.si.əm aɪˈəʊ.deɪt/ |
| Preferred IUPAC name | Calcium diiodate |
| Other names |
Calcium diiodate Iodic acid, calcium salt Calcium iodate(V) Lopac-C-1212 |
| Pronunciation | /ˈkæl.si.əm aɪˈəʊ.deɪt/ |
| Identifiers | |
| CAS Number | 7789-80-2 |
| Beilstein Reference | 676393 |
| ChEBI | CHEBI:3733 |
| ChEMBL | CHEMBL1200908 |
| ChemSpider | 54834 |
| DrugBank | DB11093 |
| ECHA InfoCard | ECHA InfoCard: 100.013.904 |
| EC Number | 231-194-7 |
| Gmelin Reference | Gmelin Reference: **83360** |
| KEGG | C18697 |
| MeSH | D017793 |
| PubChem CID | 24598 |
| RTECS number | FX9172000 |
| UNII | 9Q9ZZM0CBM |
| UN number | UN1457 |
| CAS Number | 7789-80-2 |
| Beilstein Reference | 821225 |
| ChEBI | CHEBI:32599 |
| ChEMBL | CHEMBL1201571 |
| ChemSpider | 54844 |
| DrugBank | DB11310 |
| ECHA InfoCard | ECHA InfoCard: 03-2119969268-23-0000 |
| EC Number | 233-288-5 |
| Gmelin Reference | Gmelin Reference: **1754** |
| KEGG | C15734 |
| MeSH | D002119 |
| PubChem CID | 24856 |
| RTECS number | FX9625000 |
| UNII | 13Y15L9T79 |
| UN number | UN1479 |
| Properties | |
| Chemical formula | Ca(IO3)2 |
| Molar mass | 389.88 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Densit: 4.52 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -1.37 |
| Vapor pressure | Negligible |
| Basicity (pKb) | 12.00 |
| Magnetic susceptibility (χ) | `-74.0·10⁻⁶ cm³/mol` |
| Refractive index (nD) | 1.833 |
| Dipole moment | 0 D |
| Chemical formula | Ca(IO3)2 |
| Molar mass | 389.886 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 4.28 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -0.7 |
| Vapor pressure | Negligible |
| Basicity (pKb) | pKb ≈ 12.07 |
| Magnetic susceptibility (χ) | -64.0e-6 cm³/mol |
| Refractive index (nD) | 1.834 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 174.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -857.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -738.9 kJ/mol |
| Std molar entropy (S⦵298) | 174.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -925.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1072.2 kJ/mol |
| Pharmacology | |
| ATC code | A12CA04 |
| ATC code | A12CA01 |
| Hazards | |
| Main hazards | Oxidizing solid, may intensify fire; harmful if swallowed; may cause skin and eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Store in a dry place. Store in a closed container. Dispose of contents/container in accordance with local/regional/national/international regulations. |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 4550 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1550 mg/kg |
| NIOSH | KWG93650 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Calcium Iodate: "10 mg/m3 (as nuisance dust, total dust) |
| REL (Recommended) | 0.75 mg/kg |
| Main hazards | Oxidizing solid, may intensify fire; harmful if swallowed or inhaled; causes eye, skin, and respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Store in a dry place. Store in a closed container. Dispose of contents/container in accordance with local/regional/national/international regulations. |
| NFPA 704 (fire diamond) | 2-0-0-OX |
| Lethal dose or concentration | LD50 (oral, rat): 11,500 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral (Rat): 791 mg/kg |
| NIOSH | KW3850000 |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 240 mg/kg |
| Related compounds | |
| Related compounds |
Calcium chloride Calcium hypochlorite Potassium iodate Sodium iodate Iodic acid Calcium iodide |
| Related compounds |
Calcium chlorate Calcium bromate Potassium iodate Sodium iodate |

