Calcium Glycine: Bridging Science and Industry
Historical Development
Scientists first explored amino acid chelates in the race to improve nutrient delivery and stability during the mid-to-late 20th century, and among these, calcium glycine drew a good deal of attention. Early research focused mostly on minerals like iron and zinc, but the nutritional and technical needs of the food and supplement industries drove exploration into the more stable and absorbable forms of calcium. Chemists reached for glycine, the simplest amino acid, as a chelating agent, discovering that its small size and neutrally charged structure helped it form tight bonds with calcium ions. The discovery of calcium glycine transformed calcium supplementation, especially when regular salts performed poorly in terms of bioavailability and gastrointestinal comfort. Patents started to appear in the 1980s. Over time, the improved understanding of chelation chemistry empowered manufacturers to consistently produce a calcium compound that could outperform calcium carbonate and citrate, both in solubility and in patient acceptance.
Product Overview
Calcium glycine sits at the crossroads between the worlds of nutrition science and industrial chemistry. Producers offer it as a white crystalline powder, with solid stability, mild taste, and a good safety profile. It attracts food technologists, pharmacists, and animal feed formulators with its promise of better mineral delivery and its flexibility in formulations. In supplements, calcium glycine helps minimize the chalkiness or heavy flavors that sometimes repel people from adhering to their supplement routines. In food fortification, its favorable solubility adds value for dairy alternatives, beverages, and infant formulas. Thanks to its specific molecular design, it also avoids the typical binding problems seen with other amino acids or mineral salts. Calcium glycine is more than a menu line item — it represents decades of scientific fine-tuning aimed at doing a simple job: giving the body useable calcium without the baggage of constipation, poor taste, or poor absorption.
Physical & Chemical Properties
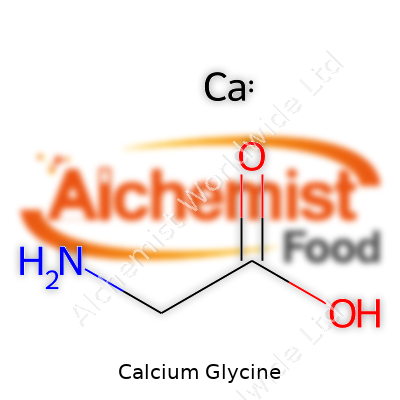
Calcium glycine shows up as a crystalline or powdery white substance, almost odorless and only faintly sweet to the taste. It dissolves reasonably well in water, with better solubility at lower pH, giving formulators an edge in product development. Chemically, it bears the formula C4H8CaN2O4. One molecule of calcium binds to two donor atoms from glycine, forming a stable ring structure. Its melting point ranges above 250°C, indicating a compound that can weather the rigors of spray-drying or tableting without unwanted breakdown. Its stability in solution removes a hurdle seen in many mineral salts, which can precipitate or form insoluble complexes. Owing to its polar characteristics, calcium glycine mixes readily with aqueous systems, sidestepping the sedimentation and taste obstacles that typify many calcium supplements.
Technical Specifications & Labeling
Commercial calcium glycine comes in various purities, usually above 95%. The specification sheet for the food industry, in my experience, will include calcium assay figures (21-23% by weight), loss on drying limits (below 5%), and heavy metal thresholds tightly regulated to guarantee safety. Technical documentation always includes information on particle size, microbial load, and absence of pathogens. Labels for dietary supplements typically call it “calcium bisglycinate” or “calcium glycine chelate,” displaying calcium content in milligrams per serving directly on the package, as required by food regulations in Europe and North America. Manufacturers often validate the identity using infrared (IR) or nuclear magnetic resonance (NMR) spectra, results of which are sometimes included in the product dossier. Transparent labeling instills consumer confidence, particularly among those looking for “chelates” instead of basic salts.
Preparation Method
The most straightforward way to make calcium glycine in large quantities uses a reaction between calcium salts and glycine under mild aqueous conditions. In a typical setup, food-grade calcium carbonate or calcium hydroxide dissolves in purified water, forming a slightly basic suspension. Glycine is added gradually with thorough agitation. The solution is stirred and kept at a narrow pH range to encourage chelation rather than precipitation. Temperature and pH play critical roles — if the pH climbs too high, competing species form; too low, and the chelation becomes inefficient. Once the reaction completes, the clear solution can run through a series of filtration, concentration, and spray-drying steps, yielding a fine powder. This approach is scalable and minimizes the risk of unreacted by-products or off-flavors. Some manufacturers go further and optimize the glycine-to-calcium ratio, using titration curves and HPLC analytics to confirm that the reaction reaches full chelate formation.
Chemical Reactions & Modifications
Most chemists leverage the zwitterionic nature of glycine to bind calcium efficiently, generating a five-membered chelate ring structure. Besides classic salt formation, researchers have tried coupling glycine with other functional groups before chelation to tune properties such as solubility, taste, or shelf stability. Under acidic conditions, calcium glycine can undergo hydrolysis, reversing to release free glycine and calcium ions. This reversibility plays to its advantage in the digestive tract, where stomach acid helps break the chelate bond, allowing for smooth absorption. Some advanced work looks at incorporating calcium glycine into layered or complexed matrices for slow-release applications, but most industrial use cases favor the simple, direct chelate. Laboratory tests have also explored using calcium glycine as a catalyst or reactant in specialty organic syntheses, but these are mostly research curiosities rather than mainstream industrial applications.
Synonyms & Product Names
In commerce and scientific parlance, calcium glycine goes by several names. The most common label is “calcium bisglycinate,” though “calcium diglycinate,” “calcium chelated with glycine,” or “glycine calcium salt” also appear in technical datasheets. Some international regulatory agencies refer to it as “calcium amino acid chelate (Glycine),” especially in food additive databases. Branded versions often put the manufacturer’s stamp on the package, but the underlying chemistry doesn’t change. End-users, especially in nutrition or pharmaceuticals, often look for phrases like “chelated calcium” as a signal of superior absorption. This nomenclature cluster sometimes confuses buyers, especially those who aren’t chemical specialists, so responsible suppliers always provide composition data and structural diagrams to distinguish true glycine chelates from blends or physical mixtures of glycine and calcium salts.
Safety & Operational Standards
The broad adoption of calcium glycine owes a lot to its robust safety profile. Acute and long-term studies in both humans and animals show it does not provoke serious side effects at typical intake levels, provided the product meets quality standards. Production facilities run frequent audits to test for contamination, trace metals, and residues from synthesis. Regulatory bodies like the US FDA, EFSA, and China’s National Health Commission recognize calcium glycine as a safe ingredient or additive, imposing strict purity and microbiological limits. Factory floors schedule regular staff training on powder handling to minimize dust inhalation, listing personal protective equipment (PPE) like masks and gloves as a baseline precaution. Packaging lines track and trace batches using blockchain or digital tracing systems, ensuring that any quality issues can quickly trigger recalls across the supply chain. Good Manufacturing Practices (GMP) govern all these checkpoints to protect end users and uphold public trust.
Application Area
My years in food technology have shown me that calcium glycine’s flexibility serves a broad set of needs. Nutritional supplements stand as the primary area, where improved absorption and fewer side effects often define consumer choices. Functional beverages, protein bars, vegan dairy replacements, and breakfast cereals all draw on calcium glycine to achieve label claims without sacrificing taste or mouthfeel. In pharmaceutical applications, it enters oral preparations for children, older adults, and individuals with absorption disorders. Veterinarians and animal nutritionists include it in advanced animal feeds, targeting bone health and reproductive health for pets and livestock. In agriculture, specialty formulations sometimes use chelated calcium to treat crop nutrient deficiencies, given its stability and ease of uptake in foliar sprays. The steady demand in these sectors pushes manufacturers to refine purity, granulation, and blending strategies without pricing themselves out of the market.
Research & Development
Curiosity never rests in the science of calcium glycine. Current R&D efforts frequently dig into improved absorption studies, using tracer isotopes to follow how effectively the chelated form crosses the gut barrier compared to salts. Food scientists investigate particle engineering, looking for ways to blend calcium glycine seamlessly into demanding matrices like acidified juices or sports shakes. Pharmacologists examine its effect on bioavailability for patients with low stomach acid or those taking medications that block calcium uptake. Formulation research explores microencapsulation or use in novel delivery systems such as gels or oro-dispersible films. Teams also run comparative taste and texture panels to document whether consumers notice subtle improvements in finished products. On top of this, global regulatory harmonization remains a moving target, as researchers gather more toxicological and clinical data to support broader product registrations.
Toxicity Research
The toxicity profile of calcium glycine sets it apart from many older calcium compounds. Acute toxicity studies in rodents using high oral doses show only mild, reversible gastrointestinal effects at vast multiples of daily recommended intakes. Human clinical studies report no significant adverse events when dosed within regulatory limits. The structure of glycine as a non-essential amino acid means it already has a central spot in basic metabolism, lowering the likelihood of surprising side effects. Long-term feeding studies in animals and occasional case reports from clinical practice reinforce the notion of high safety margins. Regulatory agencies such as EFSA place calcium glycine on allowed lists with strict maximum limits for impurities. Still, vigilance remains crucial — suppliers must routinely check for residues of starting materials, microbial contamination, and mislabeling. Without these checks, contamination or misadventure in facility hygiene could swiftly undermine decades of safety data.
Future Prospects
Looking ahead, calcium glycine will almost certainly see further expansion as more consumers and institutions demand better, science-backed nutrition in everything from emergency meal kits to specialty hospital feeds. The rise of plant-based diets, food fortification, and clinical nutrition all point to a growing need for highly bioavailable, palatable calcium sources. Scientific advances are likely to unlock custom formulations, perhaps pairing glycine chelates with other micronutrients or embedding them in advanced food structures for proactive health management. Technical teams eye greener and more efficient synthesis, possibly using biocatalysis or waste glycine streams from other industries. Tighter regulatory alignment across markets can smooth the way for global brands to unify formulation and labeling, boosting transparency and consumer acceptance worldwide. Experience suggests that companies who invest now in evidence-driven development and traceable supply chains will define the next era for chelated minerals, with calcium glycine taking center stage on ingredient panels and in the fight for better public health.
What are the health benefits of Calcium Glycine?
Why Calcium Matters for Everyday Life
Most people think about bones when they hear calcium, and for good reason. Without enough of it, bones become weak, break more easily, and osteoporosis creeps up as people age. But there’s a twist—how the body absorbs and uses calcium makes all the difference. Foods rich in calcium often come with other nutrients, but sometimes poor absorption limits the benefit. This is where calcium glycine steps in with a new approach.
What Sets Calcium Glycine Apart
Calcium glycine belongs to a group of nutrients called chelates—basically, calcium bound to glycine, an amino acid the body already knows well. This pairing creates a sort of nutritional shortcut. It helps the body pick up calcium more easily and send it where it’s actually needed. Compare that to the chalky calcium carbonate tablets that often upset stomachs—more of that calcium ends up in the toilet than working inside your body.
Better Absorption, Less Stomach Trouble
From years of watching what works for people dealing with calcium deficiency—especially women after menopause—absorption trumps high dosages every time. Research backs this up: calcium glycine absorbs in the small intestine with less interference from other minerals. Fewer people complain about bloating or constipation. Athletes, vegans, and those with lactose intolerance tend to tolerate it well. The key here is the gentle journey from supplement to bloodstream.
Protecting More than Just Bones
Calcium helps send signals along nerves, keep muscles steady, and maintain a regular heartbeat. Glycine doesn’t play a background role either. It calms nerve activity and helps rebuild damaged tissue, which matters for recovery after injuries or heavy workouts. Some studies from the past five years point to better sleep and even milder anxiety in adults using supplements with glycine. Adding glycine to calcium seems to amplify these effects, although most of these benefits need more research.
Everyday Choices to Support Health
People shouldn’t chase trendy supplements without thinking about the rest of their diet. Leafy greens, almonds, and dairy products still stack up well, but if there’s a gap that food can’t cover, calcium glycine appears as a smarter backup. People with kidney issues, young children, and pregnant women should keep their doctor in the loop since too much calcium can cause problems too.
Safe Supplementation and What to Look For
Supplements from reputable brands, clear labeling, and dosing that matches daily needs all play a big role. A daily amount between 500 and 1000 mg is what most studies recommend for adults, divided into smaller intakes for better absorption. Reading the label and knowing where the ingredients come from protects against heavy metal contamination or misleading marketing. The Food and Drug Administration doesn’t vet every supplement, so buyer beware always matters.
Moving Forward with Better Nutrition
Instead of going with the old “more is better” mindset, calcium glycine teaches a lesson about quality and synergy. Thinking about what the body can use—not just what goes in—leads to better health outcomes. Experience, research, and good choices all point toward this blend of calcium and glycine as an option worth considering.
Is Calcium Glycine better absorbed than other calcium supplements?
Calcium gets plenty of attention in health circles, with good reason. Bones, teeth, muscle contraction, nerve function—none of that works well without enough calcium. The problem isn’t always getting calcium into your body; it’s making sure your body actually absorbs it. Supplement shelves fill up with options: calcium carbonate, citrate, gluconate, and a newer name, calcium glycine.
Understanding Calcium Glycine
Calcium glycine uses glycine, an amino acid, to bond with calcium. The idea here is simple—our gut takes in amino acids more easily than some minerals alone, so pairing calcium with glycine is supposed to help the body use more of what’s swallowed. Some supplement manufacturers push this as a game-changer for folks dealing with low calcium absorption, especially older adults or those on acid-reducing medications.
How Calcium Absorption Works
My own family tried out different supplements, steering away from chalky tablets. I’ve seen first-hand that some forms upset the stomach more than others. Digestion isn’t a straight shot—absorption gets tricky with age, medications, and even what you had for breakfast. Calcium carbonate, a common choice, depends on stomach acid for breakdown. People with low stomach acid, including many over 50, tend to get less from carbonate. Calcium citrate skips this step, breaking apart more easily, and it’s long been the go-to for anyone on heartburn meds.
Calcium glycine is rolling in as a contender thanks to research showing amino acid chelates (this just means it's bound to an amino acid like glycine) get through the gut more smoothly. A study in “Biological Trace Element Research” found calcium glycine chelate absorbed more efficiently and triggered higher blood calcium levels compared to carbonate and gluconate in lab animals. Human data is growing, too—some compare these results to the time when doctors first realized vitamin D helps with calcium uptake. Early trials suggest better absorption, fewer digestive complaints, and steady blood calcium in people who take this form, especially those who historically needed to take higher doses to get the same result.
Real-World Use and Limitations
Calcium glycine can cost more, and it’s still not as common on store shelves as citrate or carbonate. Insurance doesn’t usually touch it unless prescribed. Large, long-term studies are limited. Right now, most of the data comes from short-term studies or animal models, so it’s wise to keep an eye open for more unbiased, peer-reviewed human trials before making major promises.
Supporting Healthy Bones Beyond Supplements
Any pill only does so much. Getting enough vitamin D from sunlight or supplements matters for absorption, and so does protein and weight-bearing activity. Not all calcium supplements agree with every stomach. I’ve worked with patients who tolerate calcium glycine much better—no bloating, no constipation, even at higher doses—so there’s real promise here. Keeping bones healthy means looking beyond bone density scores: a diverse diet and regular movement matter even more. Anyone curious about a new supplement, especially those with health conditions, needs to check with a healthcare professional who can run through risks and benefits.
The Path Forward for Calcium Glycine
The science behind amino acid chelates like calcium glycine lines up with what we know about bioavailability—they just fit better into natural digestive processes. People struggling to get enough calcium from food or those dealing with upset stomachs after traditional pills might find relief here. The conversation shouldn’t just circle back to what absorbs “best.” Instead, it’s worth focusing on a plan that keeps calcium levels strong without side effects, fits into daily life, and supports everything that healthy bones need—sun, strength, food, and sometimes, the right supplement.
Are there any side effects of taking Calcium Glycine?
Why Calcium Glycine Gets Attention
Supplements like calcium glycine pop up more and more often, promising convenient ways to fill dietary gaps. My doctor once raised an eyebrow when I asked about adding it alongside my regular multivitamin. He rattled off the list of nutrients Americans often skimp on, and calcium landed near the top. Dairy doesn’t suit everyone, so powder or capsule options show up on the shelves, and calcium glycine — the chelated form where glycine binds with calcium to help the body take it in — is one of them.
Possible Side Effects: Not Always Smooth Sailing
No pill works like magic. The hope is that calcium glycine won’t upset your stomach like plain calcium carbonate sometimes can. People like me with a sensitive gut often hear that chelated minerals might feel gentler. That said, too much calcium in any form starts bringing its own set of headaches. More isn’t always better.
Digestive disturbances top the list. I’ve experienced it myself after a few days of extra calcium: bloating, a bit of nausea, sometimes constipation. High doses seem to make matters worse, and others report the same thing. It happens because the body only handles a certain amount at once. A fact sheet from the National Institutes of Health points out that adults shouldn't go above 2,000–2,500 mg of calcium from all sources per day. Go over that line, and the odds of side effects only grow.
Calcium and Other Nutrient Balances
One part of this that stumps a lot of people: too much calcium can mess with how your system absorbs things like iron, zinc, and magnesium. I learned this the hard way during a stretch of marathon training, when my iron numbers slipped out of the healthy range. Big doses of calcium eaten at the same time as iron-rich meals may block some of that iron. Nutritionists often mention that point, yet supplement bottles rarely spell it out.
Long-Term Risks: Kidneys Get Involved
There's another angle to keep in mind. Feeding the body more calcium than it wants can strain the kidneys. Kidney stones sometimes run in families, and adding high doses of calcium — even in gentle forms like calcium glycine — stacks the odds if you're already at risk. A study in the New England Journal of Medicine underscores that link, especially in people who don’t drink much water or have a family tendency toward stones.
Safer Ways to Supplement
For people thinking about using calcium glycine, a blood test gives a clear read on where your levels stand. It’s easy to grab a bottle on impulse, but medical advice helps shape a safer plan. Spreading out doses and taking them with meals keeps absorption manageable and fends off side effects. Tuning into your own family background and medical history saves a lot of stress later.
Practical Steps Forward
Professional groups such as the National Osteoporosis Foundation urge people to go for dietary sources of calcium whenever possible. Yogurt, leafy greens, or fortified juices go farther than any pill—plus, they bring fiber and other nutrients along. If relying on supplements, keep an honest tally of the calcium you get from your total diet, not just the capsule.
All in all, supplements fill real gaps, but side effects exist even for “gentle” forms. Listening to your body and checking in with a healthcare provider puts you in the driver’s seat, not the vitamin aisle.
How should Calcium Glycine be taken for best results?
Why Quality and Consistency Matter
Choosing a calcium supplement can feel overwhelming. I’ve stood in supplement aisles, scanning labels, and wondered if the latest amino acid chelate forms actually deliver better results. Calcium glycine, a chelated form, grabs attention because it often absorbs better than basic calcium carbonate or citrate. The trick is that absorption counts for nothing if consistency drops or if your body isn’t ready for what you feed it.
Recent studies and the experiences of real people paint a clear picture: calcium glycine gives better bioavailability when taken alongside a basic meal—not on an empty stomach, not tossed back with coffee, not squeezed between erratic snacks. Food, especially with a bit of fat, lets your digestive system operate how it’s meant to, easing that mineral through absorption pathways. Protein, particularly, can help your gut work with glycine, nudging more calcium into your system.
How Timing and Diet Drive Results
Some friends tried calcium on an empty stomach hoping for faster results, but they complained of queasiness. Digestion seems smoother when calcium glycine slips in with breakfast or dinner. I’ve found the evening meal supports calmer digestion, maybe because I slow down and actually chew my food.
It’s not just about when, though. My nutritionist always stressed vitamin D’s critical role. If your vitamin D levels sit too low, your calcium absorption drops off. Food sources help, sunlight helps, and a blood test removes any guessing. Don’t ignore magnesium either; these minerals work together. If you only bump up calcium and forget the rest, you might struggle to see the improvements you want.
Tablet, Capsule, or Powder?
From my experience, many folks prefer capsules since they’re easy to swallow and slower to break down. I’ve tried powders when mixing into morning smoothies, and the taste stays mild, not chalky. Always check for third-party testing—purity should never be a question. Unregulated fillers and undeclared ingredients still turn up in some brands.
Watch Out for Absorption Blockers
A big glass of milk might seem natural, but too much calcium at once can overwhelm your body. If you go heavy on iron supplements at the same time, the two will compete. Spread intake across the day if you need higher doses. Caffeine, as much as I love coffee, cuts into absorption if taken together. Space them by at least an hour.
Don’t Go Overboard
It’s easy to lose track and pile supplement after supplement. My doctor flagged that more isn’t always better; kidney stones and heart strain don’t make for a better life. Track how much calcium already comes from your daily meals. Most health pros aim for total daily intakes that include food calcium, landing in the right range for your age and needs.
Thinking Bigger Than Bone Strength
For a long time, calcium just meant bones and teeth. But research links it to muscle function, heart rhythm, even mood. Sufficient calcium means more energy for day-to-day life. Still, the best supplement works only when matched with a real look at your lifestyle—meals, movement, sun, sleep, and all the rest. That awareness sticks longer than any pill.
Is Calcium Glycine safe for children and pregnant women?
Understanding Calcium Glycine
Calcium glycine isn’t as familiar a supplement as traditional calcium carbonate or calcium citrate. It combines the mineral calcium with glycine, an amino acid, to form a compound that gets absorbed easily in the gut. Calcium plays a key role in strong bones, healthy teeth, nerves, and muscle function. Safe calcium intake becomes even more crucial for kids and women during pregnancy, times when bodies are growing or carrying extra demand.
What Science Says About Its Safety
Clinical studies on calcium glycine lag behind research on time-tested options like calcium carbonate. Still, early findings suggest this new form could cause fewer stomach troubles than some traditional calcium supplements. The body recognizes glycine easily; when calcium tags along, absorption can improve. For mothers-to-be, that means a chance at better nutrition without bloating or discomfort.
Children need steady calcium, especially between ages four and 18, since bone-building happens fast during those years. For pregnant women, the need ramps up. The World Health Organization recommends extra calcium to lower the risk of complications like preeclampsia. Most safe calcium supplements come with proper labeling and reliable testing for purity and dose.
Risks and Considerations
No supplement comes risk-free. Calcium glycine has low toxicity when taken at usual doses, but side effects (like with any calcium source) stack up if too much piles on: kidney stones, constipation, interference with iron or zinc. Quality of raw materials also matters. Some cheaper products cut corners, leading to possible contamination or impurities. The FDA doesn’t check every bottle on the shelf, so parents and expecting women should stick to well-known brands with solid quality control processes.
Doctors recommend calcium based on diet, age, and condition. Some kids might have enough through dairy, leafy greens, and fish. Others might not, especially if allergies or diet restrictions get in the way. Pregnant women with lactose intolerance or vegan diets might need to supplement more, making a well-absorbed and gentle calcium source appealing.
Guidance and Practical Steps
Safety comes down to a simple rule: never guess dosage alone. Doctors and nutritionists help figure out real needs, factoring in food intake, any health issues, and medications that might get in the way. Blood tests show if levels fall short or climb too high.
Check unfamiliar labels for batch numbers and certifications. Reliable supplements share lab results and stick close to recommended dietary allowances. Keep bottles out of kids’ reach and stick to their own age-appropriate doses. Pregnant women should always talk to their OB-GYN about any supplement before buying in bulk.
Choices and Solutions
Sticking to a food-first approach works best. Think yogurt, cheese, canned salmon, broccoli, and fortified orange juice. For those who need help filling in the gaps, calcium glycine gives one more option, especially for sensitive stomachs. This form isn’t a miracle fix, but current evidence says it fits safely into most routines if used responsibly.
Each person’s situation looks a little different. Open discussion with healthcare professionals, clear information from brands, and steady attention to real needs make calcium glycine a tool, not a shortcut. With a little extra care, families can keep growing bones strong and pregnancy complications low stress.
| Names | |
| Preferred IUPAC name | calcium 2-aminoacetate |
| Other names |
Calcium bis(glycinate) Calcium diglycinate Calcium amino acid chelate Calcium glycinate |
| Pronunciation | /ˈkælsiəm ˈɡlaɪsiːn/ |
| Preferred IUPAC name | Calcium 2-aminoacetate |
| Other names |
Calcium Bisglycinate Calcium Glycinate Calcium Aminoacetate Calcium Glycine Chelate |
| Pronunciation | /ˈkæl.si.əm ˈɡlaɪ.siːn/ |
| Identifiers | |
| CAS Number | 35947-07-0 |
| Beilstein Reference | 1362124 |
| ChEBI | CHEBI:86464 |
| ChEMBL | CHEMBL1356 |
| ChemSpider | 64756 |
| DrugBank | DB11348 |
| ECHA InfoCard | ECHA InfoCard: 100.130.163 |
| EC Number | 200-306-6 |
| Gmelin Reference | 65781 |
| KEGG | C01321 |
| MeSH | D019275 |
| PubChem CID | 16211235 |
| RTECS number | MF4309000 |
| UNII | 4FGQ3S0N51 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID3021324 |
| CAS Number | 35947-07-8 |
| Beilstein Reference | 1906663 |
| ChEBI | CHEBI:86345 |
| ChEMBL | CHEMBL3305936 |
| ChemSpider | 8245814 |
| DrugBank | DB11348 |
| ECHA InfoCard | ECHA InfoCard: 100.237.875 |
| EC Number | 200-306-6 |
| Gmelin Reference | 525382 |
| KEGG | C02335 |
| MeSH | D012088 |
| PubChem CID | 71484790 |
| RTECS number | MB4150000 |
| UNII | V29W62NS9V |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID2021727 |
| Properties | |
| Chemical formula | Ca(C₂H₄NO₂)₂ |
| Molar mass | 188.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 0.8 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.14 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.10 |
| Basicity (pKb) | 7.5 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.570 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.39 D |
| Chemical formula | Ca(C₂H₄NO₂)₂ |
| Molar mass | 142.15 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.60 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.67 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.2 |
| Basicity (pKb) | “2.34” |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.47 |
| Dipole moment | 2.39 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 171.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1604.9 kJ mol^-1 |
| Std enthalpy of combustion (ΔcH⦵298) | -1607.5 kJ/mol |
| Std molar entropy (S⦵298) | 151.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1537.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1670.1 kJ/mol |
| Pharmacology | |
| ATC code | A12AA13 |
| ATC code | A12AA13 |
| Hazards | |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | Corrosive, Health hazard |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Wear protective gloves/eye protection. Avoid breathing dust. Wash thoroughly after handling. If in eyes: Rinse cautiously with water for several minutes. |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 (Oral, Rat): > 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose) for Calcium Glycine: >5000 mg/kg (rat, oral) |
| NIOSH | Not established |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 1000 - 1300 mg/day |
| IDLH (Immediate danger) | Not established |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07, GHS08, Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Do not ingest. Use only with adequate ventilation. In case of contact, rinse immediately with plenty of water. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD₅₀ (oral, rat): >5000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Calcium Glycine: 7,930 mg/kg |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 1000-1300 mg/day |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Calcium chloride Glycine Calcium gluconate Calcium citrate Calcium lactate |
| Related compounds |
Glycine Calcium chloride Calcium gluconate Calcium carbonate Calcium lactate Magnesium glycine chelate |

