Calcium Gluconate: A Look Beneath the Surface
Historical Development
Long before pharmacy shelves stocked rows of calcium supplements, calcium gluconate carved a path through medical history as an essential mineral support. Alfred Einhorn synthesized it in the late 19th century, targeting safer calcium therapy compared with its more caustic cousin, calcium chloride. Hospitals quickly embraced the compound, especially after physicians reported fewer tissue injuries during injections. By the mid-20th century, clinicians leaned on calcium gluconate as the intravenous workhorse in emergencies like hypocalcemia and magnesium sulfate toxicity. Modern production scaled up thanks to reliable fermentation techniques, bringing medical-grade purity into focus—a priority regulatory bodies continue to enforce.
Product Overview
Calcium gluconate lands on the market typically as a white, odorless crystalline powder or as a sterile solution for injection. It sees heavy use in both oral and intravenous forms, adapting to clinical needs and over-the-counter calcium supplementation. Pharmacies supply tablets, IV ampoules, syrups, and topical gels, each form shaped by safety profiles and administration routes. Pharmaceutical quality standards keep tabs on impurities, microbiological levels, and excipient compatibility, resulting in a product that meets stringent pharmacopeial demands.
Physical & Chemical Properties
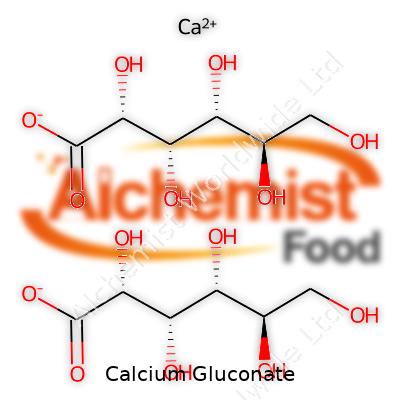
It takes the form of a colorless to white, odorless powder, highly soluble in boiling water and faintly soluble in cold water, but it barely dissolves in organic solvents. The molecular formula reads C12H22CaO14 with a molar mass of about 430 g/mol. Calcium gluconate melts close to 201°C with decomposition, which means storing and preparing solutions demands attention to temperature controls. Chemists respect its neutral to slightly acidic pH in solution, an important factor when mixing with other drugs during hospital infusions.
Technical Specifications & Labeling
High-grade calcium gluconate meets purity thresholds set by the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.), which insist on a minimum 98.5% assay content. Labels on pharmaceutical vials or bottles detail the calcium ion content, total volume, expiration date, lot number, preservatives (if any), and manufacturer credentials. Vials for intravenous use spell out sterility, pyrogen status, and specific handling instructions. Newer labeling trends focus on QR codes for instant traceability, reflecting the industry’s push toward digital safety verification.
Preparation Method
Most manufacturers source gluconic acid by aerobic fermentation of glucose with microorganisms like Aspergillus niger, followed by neutralization with calcium carbonate or calcium hydroxide. Chemical engineers optimize pH, temperature, and agitation in stainless-steel vessels to push yields upward. After filtering out biomass and excess reagents, the mixture undergoes crystallization, washing, centrifugation, and drying. The resulting solid matches pharmaceutical quality through rigorous testing for heavy metals, residual solvents, and microbial contamination.
Chemical Reactions & Modifications
Calcium gluconate dissolves readily in water and interacts with acids, generating gluconic acid and other calcium salts under right conditions. Its carboxyl and hydroxyl functional groups allow some derivatization for research—chemists tweak these moieties to probe new delivery forms or compatibility with biologically active compounds. In industry, chelation with other ions or complexation aids in nutrient delivery; for example, combining with magnesium in specialty supplements. Decomposition by heat or strong acids liberates carbon dioxide, gluconic acid, and calcium ions, demonstrating behavior that matters in high-temperature or acidic environments like digestion or sterilization.
Synonyms & Product Names
The name “Calcium gluconate” shows up under several monikers: calcium D-gluconate, E578 (as food additive), and its IUPAC designation, calcium (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate. Hospital staff recognize brands like Gluconate Calcium and Kalcinate for injectables. Supplement companies push consumer-friendly trade names in grocery chains, aiming for ease of identification by patients and caregivers.
Safety & Operational Standards
Medical teams watch patient reactions closely—injections can irritate veins or tissue if not administered properly. Intravenous preparations demand sterile technique and careful monitoring of infusion rates, since too fast a bolus can trigger cardiac arrhythmias. Safety data sheets warn of potential hazards on skin or eyes; workers handling bulk powder wear gloves, lab coats, and eye shields. Food and Drug Administration (FDA) and European Medicines Agency (EMA) scrutiny ensures batch traceability and cGMP compliance at the plant level, reinforcing the supply chain’s accountability.
Application Area
Doctors reach for calcium gluconate in acute care settings—think hypocalcemia from acute pancreatitis, or antidote for hypermagnesemia caused by magnesium sulfate overdose. Emergency departments stock it for hydrofluoric acid burns, as topical or subcutaneous injection reduces local tissue destruction. Neonatal care adds it for calcium-deficient newborns, and endocrinology clinics prescribe it for conditions like parathyroid disorders. Food technologists use it as a firming agent and preservative, labeled as E578 in canned vegetables where it improves texture and shelf-life. Veterinary medicine follows many of the same protocols, providing mineral balance in large and small animals.
Research & Development
Scientists keep probing new roles—recent trials examine slow-release implants for postmenopausal osteoporosis, while others test enhanced skin penetration in topical gels for localized injuries. Biotech startups explore nano-formulations for targeted mineral delivery, aiming for better absorption and less gastrointestinal upset. Clinical teams run post-market surveillance to catch rare allergic reactions or tissue calcification from accidental extravasation. Academic groups push new analytical methods to measure calcium ion bioavailability and cross-check between generic and branded formulations.
Toxicity Research
Calcium gluconate’s safety margin stands out compared with calcium chloride, though excess dosing can result in hypercalcemia—symptoms may include nausea, confusion, or irregular heartbeats. Animal studies detail the safe upper limits, shaping dosing regimens for frail or pediatric patients. Researchers study long-term administration for potential tissue calcification or kidney stone risks, especially among renal-compromised populations. The antidote role for hydrofluoric acid burns underscores its rapid calcium ion action but also spotlights the risks of misadministration into soft tissue, prompting continual updates to clinical practice guidelines.
Future Prospects
Calcium gluconate products will keep evolving. The trend toward patient-friendly oral supplements with better flavor and digestibility could fuel wider adoption outside hospital settings. Advances in biocompatible formulations and combination therapies stand ready to push this classic agent beyond its emergency medicine roots, with researchers eyeing targeted delivery technologies and smart packaging that tracks patient compliance. Regulatory and analytical advances keep raising quality standards. As clinicians and consumers continue demanding both efficacy and safety, calcium gluconate’s legacy is far from finished.
What is Calcium Gluconate used for?
What Calcium Gluconate Actually Does
Calcium sits at the center of so much in the body. Bones, muscles, nerve function, even how our blood clots—calcium backs it all up. So if levels drop, you feel it. That’s where calcium gluconate comes in: this compound isn’t just a bottle on a pharmacy shelf. Paramedics carry it. Surgeons use it. It’s not hype; it’s everyday life-saving stuff.
Spotlight on Medical Emergencies
I grew up in a rural town and never thought twice about calcium—until a friend landed in an ER during chemo. His calcium tanked. Calcium gluconate let his heart keep beating safely. Hypocalcemia—a big word for low blood calcium—hits more often than folks realize, especially during medical treatments, after surgery, or even in some old-fashioned poisonings. When someone’s heart races wild or muscles start to twitch and cramp, that’s the signal. Get the calcium back up fast or risk real trouble.
Doctors turn to calcium gluconate because it works quickly and faithfully. Cardiac arrest caused by certain electrolyte imbalances needs it. The antidote for magnesium sulfate overdose, another ER scenario, is straight-up calcium gluconate. Touch a strong acid or hydrofluoric acid at a factory or in a garage, and you’ll want that calcium gel to stop the burn from eating deeper into the skin. The science says plain water doesn’t work for those chemical burns the way calcium gluconate does.
Why Food Isn’t Always Enough
Plenty think they get enough calcium from milk and veggies, and many do. But illness puts pressure on the body that diet alone can’t hold up. Chronic kidney disease saps calcium stores, makes the blood acidic, and leaves bones brittle. Newborns sometimes arrive without enough calcium to keep their tiny hearts and muscles stable. Intravenous calcium gluconate rises to the occasion, especially when time runs short.
Calcium supplements line store shelves, but not all forms work the same. Calcium carbonate needs acid in the stomach to break it down, making it a no-go for folks on antacids or with digestion issues. Calcium gluconate skips these hurdles. Hospitals often pick it because infusions can slot in right through an IV, avoiding the gut completely. For the elderly, frail patients, or anyone with absorption trouble, this route can save precious minutes.
Weighing Risks and Staying Safe
Like most real medicine, calcium gluconate deserves respect. Too much drives up blood pressure or throws off the heart’s rhythm. Giving the dose slowly matters, based on what my own mentors taught during nursing rotations. Blood tests guide every step, because blindly topping off calcium can cause just as much harm as letting it drop too low. That sort of care—vigilant, careful, responsive—keeps people safe.
Looking Forward: Education and Preparedness
Training makes the difference. Every ambulance and ER stocks calcium gluconate because staff know what to look for and when to use it. The next challenge lies in making sure more doctors, parents, factory workers, and even teachers recognize when a low-calcium emergency might be unfolding, or know what to reach for after a serious chemical accident. The more we talk about these real-world scenarios, the more lives calcium gluconate will continue to save.
How should Calcium Gluconate be administered?
What Calcium Gluconate Means for Patient Care
Calcium gluconate does a lot of heavy lifting in hospitals and clinics. It steps in during emergencies: someone with dangerously low calcium, people with magnesium toxicity, kids who swallowed fluoride toothpaste, or workers exposed to hydrofluoric acid. Not many folks think much about an electrolyte like calcium until things go sideways. I’ve seen cases where a quick infusion meant the difference between a simple shift and a rescue effort. Good care comes down to knowing both the drug and the person in front of you.
Why Route and Dose Matter
Most folks visualize a shot or a pill when a doctor pulls out a med. Calcium gluconate comes as a tablet or a liquid for injection. Giving the right form depends on the situation. Hospitals often give it through a vein when emergencies crop up. Oral tablets just can’t move fast enough if someone’s heart gets jumpy or muscles start to twitch from hypocalcemia. On the other hand, oral doses help when there’s a chronic, quieter drop in calcium—like with long-term kidney issues.
Doctors give the usual injectable dose slowly into a vein after diluting it. The biggest risk shows up when someone tries to rush the process or skips proper dilution. Pushing the medicine too fast can send someone’s heart into a dangerous rhythm. Accidentally letting any of the concentrated medicine leak outside the vein brings another set of headaches—pain, skin burns, tissue damage. Medical guidelines keep it concrete: dilute in normal saline, give through a clean IV site, monitor the patient every step, and don’t take your eye off the heart monitor.
Practical Steps for Safe Use
Putting safety first means attention to detail. Double-check the patient’s kidney function before giving calcium gluconate. Adjusting doses might save a patient from trouble—folks with kidney troubles can end up calcium overloaded. Most nurses and doctors agree: use a big vein and flush with plenty of saline before and after. This cuts down the chance of the drug escaping into the skin, which is one of the worst complications I’ve witnessed.
Any time calcium gluconate gets used, keep a watchful eye for symptoms that tell you things aren’t right. Slow heartbeat, warmth at the injection site, tingling, nausea—these signs mean it’s time to pause and double-check the infusion. Speaking from experience, one fast intervention can dodge a week’s worth of harm. Having resuscitation equipment and extra hands close by, just in case, isn’t being overly cautious—it’s simply good medicine.
Training and Protocols Make a Difference
Medical mishaps often trace back to poor training or unclear steps. Hospitals that keep up regular nurse and physician education on calcium gluconate see fewer problems. Posting clear posters, holding short trainings, and walking through real-life scenarios make all the difference. The American Society of Health-System Pharmacists stresses these tools and has good protocols in place, but individual attention from experienced staff keeps patients safest.
Looking Toward Fewer Complications
Smarter, more precise use of calcium gluconate always circles back to teamwork—pharmacists, nurses, techs, and doctors on the same page. Prompt lab checks for calcium and related salts help spot emerging trouble. Using infusion pumps instead of manual push can standardize the process and take out some of the risk. Hospitals open to regular team reviews often catch patterns that would slip past if everyone just stayed in their own lane.
Balancing safety and effective use doesn’t just protect patients; it helps care teams work with more confidence. Administering calcium gluconate safely shows how much every detail and every extra pause counts in the wider world of medicine.
What are the possible side effects of Calcium Gluconate?
Understanding Calcium Gluconate’s Role
Calcium gluconate often ends up in hospitals and pharmacies because it helps treat low calcium in the blood, works against some heart rhythm issues, and can even counteract magnesium sulfate overdose. Doctors sometimes give it to people after surgery or when the body needs a fast calcium boost. I’ve seen it prescribed in both emergency rooms and regular clinics, always with the idea that it restores balance during critical moments.
Common Side Effects That Catch People
No one likes feeling worse after starting treatment, even if a medication promises help. Some folks notice mild reactions. Warmth, a flushed face, or a metallic taste pop up soon after the injection starts. Nausea and a little stomach upset sometimes tag along. My own patients usually tell me about that unmistakable tingle or stinging where the nurse put in the IV.
Every now and then, people sense their heartbeats feel out of step. Rapid heart rate or a slowing pulse sometimes shows up, especially if the dose goes in too quickly. I’ve seen a few patients turn pale or tell me they feel faint, which always makes me double-check the drip rate. Unpleasant side effects often clear up when the medication is slowed down or stopped.
More Serious Reactions Require Attention
More serious side effects raise real concern. Injecting calcium gluconate into the soft tissue by mistake can cause severe pain, swelling, and even tissue damage. That’s not just a theory—complications like “extravasation” send people to surgery to clean up the mess. Allergic reactions sometimes appear as hives, trouble breathing, or swelling around the face. The look in a patient’s eyes says it all when this happens; time is precious in those moments.
Heart rhythms can get unpredictable. Too much calcium floods the system, leading to irregular heartbeats or even cardiac arrest if not monitored closely. I always keep an eye on EKG readings during treatment, because an unexpected rhythm shift can sneak up fast. Kids and older adults seem most at risk and deserve extra caution. People with kidney trouble already struggle to clear out excess minerals, so I discuss careful dosing with families when that applies.
What Causes Side Effects?
A lot depends on who receives the calcium gluconate and why. Someone with fragile veins, poor kidney function, or a bleeding disorder carries more risk compared to a healthy adult. Fast, large doses trigger more problems, especially with vein irritation or abnormal calcium levels in the body. People who use digitalis (digoxin) for heart trouble could see toxicity at lower doses.
Improving Safety and Finding Solutions
Health workers reduce side effects if they double check the dose, use the right IV line, and monitor patients closely. I use a slow drip, and I stay in the room until I see no reactions. Good communication helps—patients who call out even mild symptoms help catch trouble early. Hospitals can cut down on mistakes with better staff training, hand-written reminders, and tracking how often side effects actually happen.
Patients play a role too. Asking questions about side effects and letting the nurse know about any odd feelings right away always makes treatment safer. Families should mention all medications, allergies, and history of heart or kidney problems before the treatment starts. Education on both sides builds trust and cuts down on risk.
Can Calcium Gluconate be taken with other medications?
Understanding Calcium Gluconate Use
Doctors turn to calcium gluconate for plenty of situations—low blood calcium, certain kinds of muscle cramping, and sometimes for heart concerns. It arrives as tablets, IV injections, or even liquid. Plenty of folks who use calcium also need other daily medicine for heart health, bones, blood pressure, or diabetes. This brings up a real question—can calcium gluconate mix well with your usual meds?
Where Interactions Crop Up
Some medicine clashes with calcium. Thiazide diuretics are drugs for high blood pressure. These can push calcium levels higher than you’d want—leading to wires crossing in the blood. Digoxin, a medicine for irregular heartbeats, can mix badly with higher blood calcium, triggering dangerous rhythms. Some antibiotics, like tetracyclines and certain fluoroquinolones, latch onto calcium and form a complex your body just throws away instead of absorbing.
Even bones and vitamins fight for stomach space. People often use calcium to strengthen bones, but too much can block the effect of bisphosphonates—drugs built for osteoporosis. Thyroid medications like levothyroxine also get tripped up if taken too close together; calcium binds up this medication and slows its entry into your system.
Lessons From Real Life
A friend’s parent ended up in the ER with serious heart flutters after starting calcium gluconate on top of digoxin—something the resident doctor caught after an ECG check and a sharp review of the medication list. It was a clear reminder: drug mixes can blindside even careful adults, especially with older folks juggling many prescriptions.
It’s easy to forget that vitamins and minerals act just as strongly as prescriptions. Sometimes people buy their supplements over the counter and don’t even mention it at the doctor’s office. Pharmacists want to help, but they can only flag problems if they know what you’re actually taking.
How to Play It Safe
Spacing out medicine works well. Wait at least two hours between calcium-rich pills and those antibiotics or thyroid meds. Never double up just because you missed a dose; that’s a recipe for stomach trouble or worse.
Make sure your doctor and pharmacist know your full medicine and supplement lineup. Carry a list, snap a photo, whatever fits your routine. Ask specifically about mixing with calcium or vitamins if you’re picking up something new.
On the research side, the FDA and major clinics keep track of reports. The evidence is there—regular review of your medicine can save a trip to the hospital. A 2023 review in the British Journal of Clinical Pharmacology spelled it out: ignoring calcium drug interactions can sabotage treatment and even put lives at risk.
The Takeaway
Nobody can completely sidestep the risk of medicine mix-ups, but a little attention and a quick talk with a professional go a long way. Calcium gluconate offers major health benefits when managed right. The key remains simple: talk with those trained to spot the risks, double-check the timing, and stay honest about what lands on your nightstand.
Who should not use Calcium Gluconate?
Knowing When Calcium Gluconate Isn’t the Answer
Seeing a bottle of calcium gluconate at a pharmacy shelf feels reassuring for many. It’s a reliable go-to during certain emergencies, and doctors rely on it to treat things like low calcium, high potassium, or magnesium poisoning. Still, every medicine carries risk, and not every situation calls for this compound. From clinically significant allergies to chronic illnesses, there’s good reason for caution.
People with Allergies to Calcium Gluconate or Similar Ingredients
Allergic reactions are no joke. For those who have ever broken out in hives or had swelling from anything containing calcium gluconate, taking it again could be risky—even if the label says it’s safe. Allergies might only mean itching for some, but severe cases can send the body into shock. Life-threatening anaphylaxis stands as a red flag, and physicians keep this in mind even before suggesting it.
Individuals with Certain Heart Issues
Calcium plays a starring role in how the heart pumps and maintains a steady rhythm. For people with trouble keeping their heartbeat regular—especially those who develop a condition called ventricular fibrillation—throwing more calcium into the mix doesn’t make sense. Giving it in these situations could make an irregular rhythm worse. Research published by the American Heart Association stresses this point: targeting calcium isn’t always right for issues with heart rhythm.
Poor Kidney Function and Chronic Kidney Disease
People rely on their kidneys to filter excess minerals from blood, including calcium. For those whose kidneys have slowed down, adding more calcium can tip the balance the wrong way. I’ve talked to patients who’ve had kidney transplants or are on dialysis; they worry about what extra minerals will do, and that concern is justified. Too much calcium in the bloodstream can cause confusion, muscle weakness, or calcification in the wrong organs. Kidney doctors—nephrologists—often steer clear unless there’s a pressing reason.
Already High Calcium Levels (Hypercalcemia)
It might seem obvious, but sometimes things slip through the cracks. People already wrestling with high calcium have to steer clear of this supplement. Symptoms can feel vague at first: fatigue, constipation, thirst. Over time, bone pain, psychiatric changes, and risky heart rhythms can show up. Adding more calcium gluconate only magnifies those symptoms.
Precautions in Specific Populations
Infants, children, and older adults face higher risks from changes in mineral levels. I’ve seen parents worried about correcting a small drop in their child’s calcium, but pediatricians warn that tinkering with mineral status can have lasting consequences during growth. Seniors, too, can develop kidney and heart problems that worsen with extra supplementation. Taking a supplement just because you “read about it” doesn’t always fit individual needs.
Drug Interactions Complicate the Picture
Mixing calcium gluconate with other medications can spell trouble. For people on digoxin—a medicine for serious heart issues—high calcium intake can heighten the risk of toxic effects. Some antibiotics, like tetracyclines, bind to calcium and become useless as a result. This is why doctors and pharmacists ask about every pill, supplement, or vitamin a patient already takes.
Looking at Individual Health
Instead of grabbing a supplement on impulse, think about health as a whole. A trained provider can test blood, check kidney function, and read an EKG. They listen to symptoms that don’t always show up in numbers. These checks help shape the decision to give—or skip—calcium gluconate. The real key comes from respecting those signals and trusting a professional who knows the story behind the prescription.
| Names | |
| Preferred IUPAC name | Calcium (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate |
| Other names |
Calcium D-gluconate Gluconic acid calcium salt Calglu |
| Pronunciation | /ˈkæl.si.əm ˈɡluː.kə.neɪt/ |
| Preferred IUPAC name | Calcium (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate |
| Other names |
Glucal Calcium D-gluconate Gluconic acid, calcium salt E578 |
| Pronunciation | /ˈkælsiəm ˈɡluːkəneɪt/ |
| Identifiers | |
| CAS Number | 299-28-5 |
| Beilstein Reference | Beilstein Reference: 3855565 |
| ChEBI | CHEBI:31344 |
| ChEMBL | CHEMBL1201093 |
| ChemSpider | 54660 |
| DrugBank | DB01373 |
| ECHA InfoCard | 03e879b4-636b-4c17-81ba-b00883790913 |
| EC Number | EC 208-926-9 |
| Gmelin Reference | 16929 |
| KEGG | C00740 |
| MeSH | D002121 |
| PubChem CID | 12992 |
| RTECS number | EW4150000 |
| UNII | M87AE75M5L |
| UN number | UN2557 |
| CAS Number | 299-28-5 |
| Beilstein Reference | 1720448 |
| ChEBI | CHEBI:31343 |
| ChEMBL | CHEMBL1201190 |
| ChemSpider | 5468 |
| DrugBank | DB01373 |
| ECHA InfoCard | 03b3d84d-ad6a-431a-8987-f8c845a7bac4 |
| EC Number | E 578 |
| Gmelin Reference | 16807 |
| KEGG | C00256 |
| MeSH | D002121 |
| PubChem CID | 5342340 |
| RTECS number | FF8050000 |
| UNII | SX0N0G3YNJ |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C12H22CaO14 |
| Molar mass | 430.373 g/mol |
| Appearance | White crystalline granules or powder |
| Odor | Odorless |
| Density | DENSITY: 0.7 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -3.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.39 |
| Basicity (pKb) | 12.74 |
| Magnetic susceptibility (χ) | -72.0e-6 cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.82 D |
| Chemical formula | C12H22CaO14 |
| Molar mass | 430.373 g/mol |
| Appearance | White, crystalline powder |
| Odor | Odorless |
| Density | Density: 0.7 g/cm³ |
| Solubility in water | 3.5 g/100 mL (25 °C) |
| log P | -1.43 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 12.3 |
| Basicity (pKb) | 13.36 |
| Magnetic susceptibility (χ) | -5.1×10⁻⁶ |
| Viscosity | Viscous liquid |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2080 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3220 kJ/mol |
| Std molar entropy (S⦵298) | 330.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2175.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –2713 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A12AA03 |
| ATC code | A12AA03 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07, GHS08 |
| Hazard statements | May cause eye irritation. |
| Precautionary statements | Wash thoroughly after handling. Do not eat, drink or smoke when using this product. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 10,940 mg/kg |
| LD50 (median dose) | LD50 (median dose): 7,340 mg/kg (oral, rat) |
| NIOSH | WN3675000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Calcium Gluconate: Not established. |
| REL (Recommended) | 1000 mg |
| Main hazards | May cause irritation to the eyes, skin, and respiratory tract. |
| GHS labelling | GHS07 |
| Pictograms | GHS07, GHS08 |
| Signal word | No signal word |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | P264, P270, P301+P312, P330 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 (oral, rat): 7,420 mg/kg |
| LD50 (median dose) | 7,940 mg/kg (rat, oral) |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 1000 mg |
| Related compounds | |
| Related compounds |
Gluconic acid Calcium lactate Calcium chloride Magnesium gluconate Sodium gluconate |
| Related compounds |
Gluconic acid Sodium gluconate Calcium lactate |

