Calcium Carbonate: A Close Look at a Trusted Compound
Historical Development
Long before modern labs and precise scales, people relied on simple minerals pulled straight from the earth. Chalk carvings found across Europe, ancient Roman masonry, and even the Great Pyramids all owe some of their endurance to calcium carbonate. People learned to grind limestone for building, mixing slaked lime for mortar in construction, and using chalk as a primitive writing tool. These uses stretch back thousands of years. As science gained traction in the 18th and 19th centuries, advancements in chemistry began to isolate and define calcium carbonate, turning it from a basic building stone to a staple in medicine, industry, and research. Generations of farmers spread crushed stone for soil conditioning without knowing the chemistry unfolding in their fields. The journey of this compound charts the shift from practical folk knowledge to rigorous science, with ongoing refinements through industrialization and automation ensuring quality and reliability far beyond those early quarry days.
Product Overview
Calcium carbonate comes as a white, odorless powder or colorless crystals, found in sources like limestone, marble, and chalk. It’s easy to spot in everything from simple antacid tablets to toothpaste and baking powder. The material turns up elsewhere, bolstering plastics, papers, paints, and ceramics. Manufacturers use both ground (GCC) and precipitated calcium carbonate (PCC), tailoring the product for each purpose. Some batches get tightly controlled for particle size, brightness, or purity to serve demanding applications in coatings or food. Food-grade lots guarantee the absence of heavy metals or harmful impurities, while industrial sacks keep costs low for construction and manufacturing.
Physical & Chemical Properties
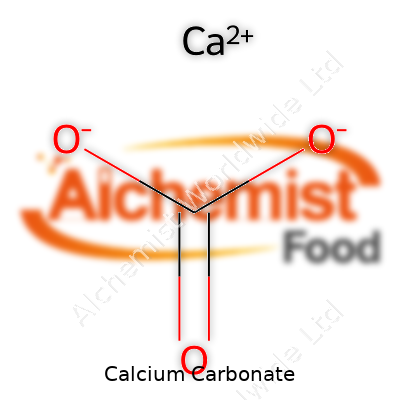
This compound holds the formula CaCO3. It resists most solvents but dissolves in acids with a signature fizzing that hints at the release of carbon dioxide. It packs in a molar mass of around 100 grams per mole and a density near 2.7 g/cm3. With a melting point above 800°C, it withstands plenty of industrial heat before breaking down. Pure calcium carbonate reflects light well and feels slightly gritty between the fingers. Despite its general stability, it doesn’t survive well in acidic settings—a fact that shapes its use as an antacid and soil amendment. Years working with it taught me to expect dusty hands and a persistent white film on any exposed surface.
Technical Specifications & Labeling
Labels matter when customers need assurance on quality, especially in the food and pharmaceutical industries. Specifications often spell out purity percentages—typically over 98%—along with limits on iron, magnesium, and other trace elements. Particle size distribution, whiteness, and moisture content show up as routine figures. Labels make clear the batch number, date of production, and certifying agencies. On a pharmacy shelf, the carton lists dosage and intended use. In a paint shop, the technical sheet highlights brightness and oil absorption numbers since these influence finish and spreadability in coatings. Plenty of debates erupt over specification details simply because product performance ties so directly to the numbers on those stat sheets.
Preparation Method
Extracting and purifying calcium carbonate begins with quarrying limestone, marble, or chalk. The raw stone is crushed and sifted to the needed size—coarse for soil, finer for plastics or paper. Precipitated calcium carbonate starts with limestone heated to produce lime (calcium oxide), then mixed with water to form slurry and bubbled with carbon dioxide. This controlled reaction generates ultra-fine, high-purity crystals whose size and surface area get tuned for applications in food, medicine, or specialty papers. Anyone working near a quarry or plant recognizes the haze, distinct clatter, and ever-present fine dust clinging to clothing at the end of a shift.
Chemical Reactions & Modifications
One of the most expected reactions links calcium carbonate with acids. Vinegar over chalk sparks bubbles as CO2 escapes—basic school chemistry at work. Industrially, that reaction comes up everywhere from the manufacture of baking powder to the neutralization of acidic wastewater. Calcium carbonate also reacts under high heat to form lime and release carbon dioxide—a key step in cement production and steelmaking. Various modifications alter its surface by treating it with stearic acid or silanes, catering to different fillers for rubber, plastics, or paints. This surface work improves mix-in with other ingredients and tweaks physical properties, like dispersion and flexibility, in finished goods.
Synonyms & Product Names
Calcium carbonate turns up under different names and trade labels. Simple terms like calcite, aragonite, marble dust, or chalk appear on bulk orders. In food and medicine, it reads as E170 or “carbonate of lime”. Commercial bags bear names tied to the stone’s origin—whitening chalk, agricultural lime, or industrial filler. Each tag signals a slightly different intended use and purity grade, driving differences in price and regulation. Choosing the right synonym sometimes makes or breaks a product shipment, especially for buyers navigating international standards.
Safety & Operational Standards
Though widely used, calcium carbonate requires respect during processing and handling. Inhalation of fine dust irritates the lungs and eyes—long histories in mining towns prove the risks of neglecting dust control. Workers receive training on respirator use and proper ventilation. Bulk handling systems rely on enclosed conveyors and dust suppression to limit airborne exposure. Food and pharmaceutical grades follow strict inspections, routine audits, and ongoing quality checks. Regulatory agencies like the FDA and WHO maintain approval lists, but any breach in operational standards triggers product recalls and costly downtime for manufacturers. Following safe practices keeps people healthy both on the plant floor and downstream in consumer goods.
Application Area
Walk down any grocery store aisle or construction site, and chances are good that calcium carbonate lurks somewhere nearby. It's standard in pills for managing stomach acid and calcium deficiency. Food processors use it for flour bleaching or as a handy firming agent in canned veggies. Paints and coatings demand it for color brightness and bulk. Papermakers rely on it for that crisp, opaque finish in modern printing stock. In agriculture, ground limestone sweetens acidic soils and boosts crop yields. Plastics and rubber producers add it to control texture, cost, and processing ease. My own experience in a small paint shop showed how adjusting the calcium carbonate grade transformed the final shade and texture of the wall finish—one batch clumped or dulled, another spread smooth and bright.
Research & Development
Current research pushes boundaries, chasing improvements in purity, particle morphology, and novel uses. Some labs aim to create tailor-made micro- and nanoparticles for targeted drug delivery or high-end electronic components. Others focus on sustainable extraction or recycling, especially using industrial by-products like boiler ash to synthesize calcium carbonate. Trials explore blends with polymer matrices or innovative coatings to deliver better barrier properties or biodegradability in packaging. Partnerships with universities draw on both academic theory and rugged real-world testing. The open questions range from optimizing mineral use in green building to cracking code on carbon capture and storage systems that take advantage of the CO2-binding power of this humble mineral.
Toxicity Research
As everyday exposure grows, toxicity research remains a key pillar of responsible use. For most people, calcium carbonate taken orally in moderate doses stays safe and effective. Overuse or chronic exposure, especially through dust in the workplace, brings risks of lung irritation or—rarely—hypercalcemia. Studies track occupational cohorts, linking high, chronic exposures to minor but real respiratory effects. Regulatory agencies review new studies on developmental impacts or rare sensitizations. Companies fund ongoing environmental testing to cover runoff or by-product toxicity. Families see reassurance when medical and regulatory consensus continues to support everyday uses, as toxicologists watch for any signals that could require tighter controls.
Future Prospects
The future of calcium carbonate looks tied to global pushes for sustainability, greener materials, and more efficient manufacturing. Prospects include closed-loop production where waste carbon dioxide feeds back into new batch synthesis, and where innovative coatings help reduce waste in packaging. As battery tech advances, researchers check if calcium carbonate nanostructures support next-generation electrodes or power cells. The construction sector eyes it for eco-friendly concrete blends, while pharma giants experiment with pH-sensitive coatings for targeted drug delivery. Everyday uses will likely expand in step with technological improvement, stricter safety norms, and demands for renewable, recyclable resources.
What is calcium carbonate used for?
Hidden in Plain Sight
Walk through a hardware store or a pharmacy, and you’ll run into calcium carbonate in more forms than you probably realize. Pick up a box of antacids, stare at a wall painted bright white, or check the label on a tablet of vitamin supplements. There it is, filling gaps most of us never think about.
Boosting Health, Easing Pain
Doctors and nutritionists invest plenty of time talking about bone health. Growing up, many have heard about the importance of getting enough calcium. Dairy and leafy greens get top billing, but supplements draw on calcium carbonate for a simple reason: it carries a large dose of absorbable calcium in a small, inexpensive package. Anyone low on calcium—kids growing fast, older adults facing bone-loss, pregnant women—leans on these supplements.
Pharmacies stock antacids with the same compound. It puts out the fire of acid reflux with a fizz and a neutralizing punch. There aren’t many things in a medicine cabinet that work as fast or as dependably. And with the right oversight, most people can safely use these tablets as needed for heartburn or occasional upset stomach.
On the Job in Construction and Industry
Step out of daily life and into a construction site or a factory, and you’ll see calcium carbonate as a quiet workhorse. Mixing cement, manufacturing glass, rubber production—these jobs depend on stability and strength. Construction-grade limestone, loaded with calcium carbonate, gives concrete its backbone. Without it, the city skylines wouldn’t reach as high, and highways would crumble sooner.
Paper mills rely on calcium carbonate to make surfaces brighter, smoother, and easier to write on. The same goes for painters: the pigment in white paint and the chalk that teachers once scraped on blackboards both spring from this mineral. In toothpaste, it scours teeth clean; in food, it keeps baking powders consistent and provides a cheap, safe way to add calcium to bread or cereal.
Tough Choices and Trade-offs
No chemical does its job everywhere without some eyebrow-raising side effects. Mining for limestone leaves scars on landscapes, and making chalk powder releases dust that can irritate lungs. Regulations help, but tighter oversight and cleaner technology reduce the impact even more. Investing in reclamation—restoring mined land for wildlife or replanting forests—carries well-documented benefits.
Choices made in one industry ripple outwards. For people with kidney trouble, too much calcium carbonate in a diet can backfire, leading to problems doctors must sort out. The same compound that fixes heartburn can, in big doses, tip the math toward kidney stones. Education and clearer labels on supplements and food help more people avoid these risks.
Looking Ahead
There’s no risk of running out of calcium carbonate; it’s one of Earth’s most abundant minerals. The big challenge lies in using it wisely. More companies are exploring low-impact mining, better recycling, and more efficient ways to make paint, pills, and paper. Each new approach shows that even common minerals deserve innovation and real-world know-how to do less harm and more good.
Is calcium carbonate safe to take daily?
Calcium in Everyday Life
Calcium plays a huge part in building bones and keeping them strong. Growing up, my family swore by dairy for healthy teeth and a sturdy frame, and most people hear about calcium for the first time from a dentist or a school nurse talking about milk. These days, plenty of folks grab calcium carbonate tablets from the store shelf, hoping to boost their intake, especially as they get older or get advice from their doctor.
Understanding What’s in the Pill
Calcium carbonate works well for folks who struggle to get enough calcium from food alone. Found in rocks and shells, this mineral ends up in common antacid tablets and over-the-counter supplements. In the United States, the Recommended Dietary Allowance (RDA) for adults hovers around 1,000 to 1,200 mg of calcium a day. Most tablets provide about 500 mg of elemental calcium. For people who don’t eat dairy or dark greens, these supplements fill the gap.
Looking at the Research
Doctors warn that too much of a good thing can backfire. Swallowing handfuls of calcium every day without checking the total intake sets the stage for kidney stones and, according to some research, may increase the risk of heart problems. A 2016 study in the Journal of the American Heart Association stirred the pot when it suggested that high dose supplements might build up deposits in arteries. That study left a lot of folks questioning their routines.
Personal experience matters here, too. In my work talking with pharmacists, I learned the body only absorbs so much calcium at a time — usually 500 mg per meal. Taking more doesn’t mean better results. It makes sense to split up doses and not rely on the pill alone.
Weighing the Ups and Downs
Calcium carbonate offers real help for people who live with low bone mass or heightened fracture risk. It’s cheap, easy to find, and the chewable forms help people who can’t swallow pills. On the flip side, people get caught up in online advice and sometimes buy unnecessary supplements that wildly overshoot their needs. The main danger doesn’t come from the supplement itself, but from ignoring overall health: a poor diet, no exercise, and too much sitting time.
Absorption drops when people gulp down calcium with iron supplements or too much caffeine. Others fight stomach issues — bloating, gas, constipation — from this form. People with kidney disease or those who take certain drugs (such as thiazide diuretics) might run into trouble if they pile on extra calcium. For these folks, a doctor’s advice beats guesswork from a bottle.
Finding a Smarter Approach
Staying informed about what goes into daily routines pays off over time. I’ve seen friends grow stronger bones by combining modest calcium, some sunlight for vitamin D, and weight-bearing exercise — all of which matter more than just popping tablets. The most useful lesson is checking total intake from both diet and supplements and talking it through with a reliable, credentialed health professional. As people age, needs change, and adjustments go a long way toward staying healthy, strong, and out of the emergency room.
What are the side effects of calcium carbonate?
Looking at What Happens in Real Life
People often pick up calcium carbonate because it’s easy to find. It’s in antacids, those chewable tablets that tackle heartburn and boost calcium intake. Doctors recommend it to folks who don’t get enough dairy or other sources of calcium. On doctor’s orders, I once added calcium carbonate to my routine for bone health. At first, things went fine. Then I began to notice a few changes—and that’s where the conversation about side effects starts to mean something real, not just words on a pharmacy insert.
What Most Folks Notice First
The gut never lies. Constipation shows up pretty frequently. Over half the people I know who take calcium carbonate long-term complain about being blocked up. The body doesn’t absorb all that extra calcium, and it slows down the intestines. This side effect is common enough that doctors and pharmacists spell it out in plain talk—too much calcium carbonate can slow your system down. Some folks also get bloated or feel like their stomach is puffed up. Nausea isn’t rare either, especially if the tablets aren’t taken with food.
Serious But Less Frequent Side Effects
Big picture, calcium forms the backbone of our bones, but it demands a careful balance. Overdo it, and trouble steps in. Hypercalcemia is what the medical textbooks call too much calcium in the blood. You’d notice it through headaches, bone aches, feeling thirsty, or even getting kidney stones. People who take high doses for a long time, especially without a doctor watching their blood levels, run this risk. Kidney stones hurt—a lot. The risk doubles if you don’t drink enough water. Some patients told me about trips to the ER with pain so sharp they couldn’t stand straight. The link between calcium carbonate and kidney stones shows up in research, too. One study in the New England Journal of Medicine found high calcium supplement intake ticked up kidney stone formation by about 20% in adults.
Beyond the Obvious
Calcium carbonate can block your body from absorbing other important stuff. People with iron deficiency might see their iron levels dip further if they combine these supplements. Some medicines, like certain antibiotics and thyroid treatments, might not work as well if you take them too close to a calcium carbonate dose. The pills act like a sponge, soaking up the medicine and making it harder for your system to benefit. I learned pretty quickly not to mix my morning supplement with my thyroid meds—and that came from personal trial and error.
Finding the Right Approach
People ask if there’s a way around these troubles. For a start, folks can drink more water and spread out their supplements with meals. Doctors sometimes suggest split doses, lower strength pills, or switching to calcium citrate to cut down on the risk of stomach issues and kidney stones. Getting calcium from food—greens, nuts, dairy—usually means fewer side effects. For most, a quick chat with a doctor or a registered dietitian helps clear up safe dosing, medication timing, and whether a supplement fits into their health plan. Smart decisions begin with real information and honest conversations about how your body reacts.
How should I take calcium carbonate supplements?
Why Bother With Calcium?
Bones matter, and real life gets busy. It’s easy to forget that they need care, too, until something cracks or aches. Calcium carbonate remains one of the cheapest, most accessible ways to step up your bone health. Researchers link enough calcium with stronger bones, fewer fractures, even steadier muscle and nerve signals. Sometimes food falls short—those who avoid dairy, live with lactose issues, or just don’t eat well all the time, miss out.
Getting Absorption Right
Calcium carbonate stands out for its price, but it asks for acid. The body absorbs this kind best when you take it with a meal or snack. Many people swallow it on an empty stomach and then wonder why it doesn't do much. The acid from food makes the difference. According to studies from the National Institutes of Health, even folks in their thirties and forties see a jump in absorption this way.
Splitting up the dose actually helps. Instead of gulping a big pill twice a day, smaller amounts with breakfast and dinner go further. The gut hits a limit, usually around 500 mg at once. Any more, and you’re just making expensive urine.
Watch for Interactions and Risks
Calcium carbonate doesn’t play well with every medicine. It can block how the body takes in iron or thyroid meds. I’ve lost count of the times pharmacists have reminded me to wait two hours between taking these. People on proton pump inhibitors for heartburn might want to switch to a different calcium source because lower stomach acid softens the benefits.
Taking too much calcium can lead to kidney stones, constipation, or, rarely, heart issues. The National Academy of Sciences suggests most adults aim for 1,000-1,200 mg of calcium a day from all sources, supplements included. Going over can tip the balance and cause more harm than good.
Don’t Skip the Vitamin D
Vitamin D puts calcium to work by helping the body pull it from the gut into the bloodstream. Without enough vitamin D—from sun, oily fish, or a supplement—swallowing all the calcium in the world won’t solve a thing. Testing vitamin D levels can show if extra support is needed, especially if tiredness or bone pain creeps in.
Food First, Pills as Plan B
Nothing beats real food. Dairy, leafy greens, almonds, tofu—they give more than just calcium. They stack in magnesium and potassium, and offer fiber. Studies show diets rich in plant and dairy calcium bring longer-term benefits than pills alone. If supplements come into play, picking trusted brands matters. The United States Pharmacopeia (USP) mark shows the supplement was tested for quality.
Better Habits, Better Bones
Bone health draws from more than just one pill. Weight-bearing exercise encourages bones to get stronger. Skipping cigarettes, keeping alcohol in check, and eating for health also affect calcium balance. Staying hydrated helps prevent kidney stones, especially if supplements are part of the routine.
Keeping it simple—take calcium carbonate with food and keep doses small. Watch for drug interactions, don’t skip vitamin D, and let food lead the way. Bones only last a lifetime if treated right.
Can calcium carbonate interact with other medications?
What Makes Calcium Carbonate Tricky
Calcium carbonate usually pops up in the medicine cabinet as an antacid. Many people chew it to get relief from heartburn. It looks harmless. But I’ve seen how it complicates other medicines. Most folks don’t realize that popping a few tablets can throw off how other pills work. This is not some rare event—it happens more often than you might think.
How Calcium Messes with Absorption
I remember talking with a neighbor who couldn’t figure out why her thyroid pills weren’t working. She always ate breakfast with coffee, her thyroid tablet, and... two chalky antacids. The calcium formed complexes in her stomach that stopped levothyroxine, her thyroid medicine, from being absorbed. She just thought the chalky tablets meant nothing. Turns out, they can grab onto certain medications, especially ones for osteoporosis, thyroid issues, HIV, and some antibiotics. The result? Lowered medicine levels and frustrated patients who feel worse even while sticking with their routines.
What Science Has to Say
This is not just one person’s experience. Research backs this up. A study in the Annals of Pharmacotherapy found that calcium carbonate can reduce the absorption of antibiotics like tetracycline and ciprofloxacin by more than half. These are drugs people rely on to get better fast. Less absorption means longer sickness or risk of the bacteria becoming resistant.
Doctors and pharmacists often see people whose blood pressure or mood medicines aren’t meeting expectations. Calcium blocks the gut from soaking in bisphosphonates, used for weak bones, and some seizure drugs. These problems don’t need fancy genetic testing or expensive labs to spot—they stem from a basic chemistry rule: calcium loves to cling to other molecules, making them too large to pass from the stomach to the bloodstream.
More Than Just a Pill Problem
For older adults who take a handful of pills every morning, the danger multiplies. Calcium carbonate may look like a simple fix for heartburn, but combine that with iron, magnesium, or certain prescription drugs and suddenly a straightforward routine gets risky. Side effects from missed or messed-up dosing creep in. Symptoms can be subtle—feeling tired, a return of reflux, bones that stay weak. The root cause gets missed unless someone asks about antacids.
Smart Solutions That Work in Real Life
The good news is that it’s simple to dodge these problems. My own experience as a caregiver and pharmacy regular taught me to spread out the timing—never take calcium with those sensitive meds. Waiting two hours before or after makes a difference. Health professionals can offer a quick review to catch the biggest risks. A conversation at the pharmacy or clinic helps clear up confusion about what to take and when.
Reading medicine labels sounds boring but it pays off. The small print sometimes lists surprising interactions. Online tools help spot risks, but nothing beats talking things over with a real pharmacist or experienced doctor. Families and caregivers should ask questions if someone feels off after starting an antacid. That simple question could sort out weeks of confusion and get people back on track.
People often want to fix heartburn or calcium deficiency fast, but mixing supplements and prescription drugs takes care. By understanding which medicines don’t mix well with calcium carbonate, you stay on track with your treatment and avoid wasting time or money chasing side effects.
| Names | |
| Preferred IUPAC name | Calcium carbonate |
| Other names |
Agricultural Lime Calcite Chalk Limestone Marble Dust Whiting |
| Pronunciation | /ˈkæl.si.əm ˈkɑː.bə.neɪt/ |
| Preferred IUPAC name | Calcium carbonate |
| Other names |
CaCO3 Limestone Chalk Calcite Aragonite Marble Whiting Ground Calcium Carbonate Precipitated Calcium Carbonate |
| Pronunciation | /ˈkæl.si.əm ˈkɑː.bə.neɪt/ |
| Identifiers | |
| CAS Number | 471-34-1 |
| Beilstein Reference | 13665 |
| ChEBI | CHEBI:3311 |
| ChEMBL | CHEMBL1201751 |
| ChemSpider | 5290 |
| DrugBank | DB06724 |
| ECHA InfoCard | EC 208-318-2 |
| EC Number | 207-439-9 |
| Gmelin Reference | 31913 |
| KEGG | C00076 |
| MeSH | D002121 |
| PubChem CID | 10112 |
| RTECS number | FF9335000 |
| UNII | H0G9379FGK |
| UN number | UN2072 |
| CAS Number | 471-34-1 |
| Beilstein Reference | 1905 |
| ChEBI | CHEBI:3311 |
| ChEMBL | CHEMBL1417 |
| ChemSpider | 5296 |
| DrugBank | DB06724 |
| ECHA InfoCard | 05eaf51b-a8a6-4d9c-9af9-866ba319ef60 |
| EC Number | 207-439-9 |
| Gmelin Reference | 378 |
| KEGG | C09809 |
| MeSH | D002121 |
| PubChem CID | 10112 |
| RTECS number | FF9335000 |
| UNII | 1KSV9V4Y4I |
| UN number | UN2072 |
| Properties | |
| Chemical formula | CaCO3 |
| Molar mass | 100.09 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 2.71 g/cm³ |
| Solubility in water | 0.013 g/L (25 °C) |
| log P | -1.37 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 9.0 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | \-23.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.658 |
| Dipole moment | 0 D |
| Chemical formula | CaCO3 |
| Molar mass | 100.09 g/mol |
| Appearance | White powder or colorless crystals |
| Odor | Odorless |
| Density | 2.71 g/cm³ |
| Solubility in water | 0.013 g/L (25 °C) |
| log P | 0.00 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 9.91 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | −22.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.658 |
| Dipole moment | 0 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 92.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1206.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1207.6 kJ/mol |
| Std molar entropy (S⦵298) | 92.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1206.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1207 kJ/mol |
| Pharmacology | |
| ATC code | A12AA04 |
| ATC code | A12AA04 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes skin and eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture. |
| Precautionary statements | Keep container tightly closed. Store in a dry place. Avoid breathing dust. Wash hands thoroughly after handling. Use only outdoors or in a well-ventilated area. Wear protective gloves/eye protection/face protection. |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Lethal dose or concentration | LD50 Oral Rat: >5000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Calcium Carbonate: "6450 mg/kg (oral, rat) |
| NIOSH | CC1925000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Calcium Carbonate: 15 mg/m³ (total dust), 5 mg/m³ (respirable fraction) |
| REL (Recommended) | 1300 mg/day |
| Main hazards | May cause respiratory irritation. |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture. |
| Precautionary statements | P264, P270, P305+P351+P338, P313 |
| NFPA 704 (fire diamond) | 0-0-0 |
| Lethal dose or concentration | LD50 Oral - Rat - 6,450 mg/kg |
| LD50 (median dose) | 6450 mg/kg (oral, rat) |
| NIOSH | CC0700000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Calcium Carbonate: 15 mg/m³ (total dust), 5 mg/m³ (respirable fraction) |
| REL (Recommended) | 1300 mg/day |
| Related compounds | |
| Related compounds |
Calcium oxide Calcium hydroxide Calcium bicarbonate Magnesium carbonate Strontium carbonate |
| Related compounds |
Calcium oxide Calcium hydroxide Calcium bicarbonate |

