Calcium Bicarbonate: From Ancient Curiosity to Modern Application
Historical Development
Long before chemistry grew into a science of elements and reactions, folks noticed that springs in certain regions left a white crust on kettles and stones. This is the earliest relationship people had with compounds like calcium bicarbonate. The water tasted different, left residue, and over time led to what everyone now calls “hard water.” Scholars of the Enlightenment dug into the problem, discovering that this residue related to specific salts dissolved by rainwater picking up atmospheric carbon dioxide and then trickling through limestone. The slow march of experiments and shared knowledge brought the hidden reactions into the light—by the late 19th century, chemistry textbooks explained how calcium carbonates form and break down, with calcium bicarbonate taking up a central role in natural water cycles and geochemistry.
Product Overview
Calcium bicarbonate doesn’t arrive on a shelf as a jar or powder. In its purest state, it stays dissolved in water, making it tricky for companies to bottle or sell directly. Most folks encounter it as part of mineral water or in the hardness of tap water, which shapes everything from taste to industrial processes requiring delicate machinery. It isn’t flashy in its appearance or marketing, but its impact stretches from municipal water treatment to commercial brewing, where controlling mineral content matters to every batch’s flavor.
Physical & Chemical Properties
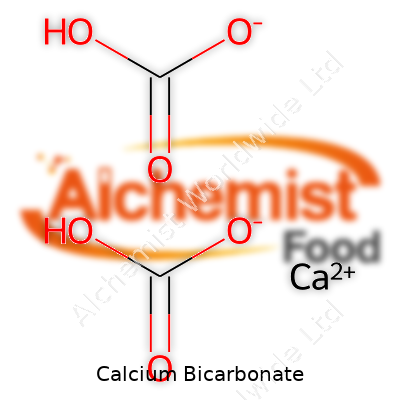
Calcium bicarbonate forms when carbon dioxide dissolves in water that contains calcium carbonate. Its chemical formula is Ca(HCO₃)₂. This compound only shows up in solution—it breaks apart as soon as water evaporates, producing insoluble calcium carbonate, CO₂, and water. It doesn’t come as a solid that you can scoop; instead, it’s a balance in the water, always at risk of tipping if the conditions change. Chemically, it serves as a weak base and interacts easily with acids and heat, which matters a lot in real-world settings from geological formation to scale buildup in plumbing.
Technical Specifications & Labeling
No company slaps a label on a bag of calcium bicarbonate granules at the hardware store—regulatory standards tie more to water quality and the measurement of hardness than to packaging labels. Analytical grades in laboratories require strict standards for purity, documented through certificates and lot numbers. Drinking water regulations, for example, monitor calcium and bicarbonate ion concentrations, which come with established maximum allowable limits set by international and local health organizations. On test reports and safety sheets, information sticks to concentration ranges, storage conditions for solutions, and methods for removal or neutralization.
Preparation Method
Producing calcium bicarbonate doesn’t involve a factory filled with mixers and vats. Instead, the process reflects the dance of nature—passing carbon dioxide through water that holds calcium carbonate, like limestone or chalk. The gas dissolves, reacts, and temporarily forms calcium bicarbonate. In labs, bubbling CO₂ through a suspension of calcium carbonate in distilled water achieves the same goal. The result remains stable as long as CO₂ levels stay up and the solution doesn’t dry out or heat up too much. Preparing it requires careful control of gas flow, temperature, and pressure. If either slides off target, the solution decomposes, and one ends up with chalky deposits or fizzing bubbles.
Chemical Reactions & Modifications
Everyone who’s boiled hard water knows about its biggest reaction. Heating a solution forces calcium bicarbonate to break down, leaving calcium carbonate deposits and releasing CO₂. Acidification with hydrochloric acid frees carbon dioxide again, sometimes used in cleaning and scale removal. Water softening tech targets these reactions, swapping calcium ions with sodium or potassium using special resins, so plumbing keeps running smooth. Geologists devote plenty of energy to these transformations, tracing the creation of stalactites and stalagmites in caves—direct evidence of nature’s slow chemical handiwork.
Synonyms & Product Names
You won’t find a supermarket shelf full of “calcium hydrogen carbonate,” but that name, along with “acid calcium carbonate,” shows up in chemical catalogs and textbooks. In the industry, workers talk about “temporary hardness” in water, technically driven by the presence of calcium bicarbonate. In brewing or agriculture, it’s all about mineral analysis and balancing these components in water to match crop or process needs. These different names reinforce how one compound weaves itself through agriculture, industry, and daily life, even if the product rarely stands out with slick branding.
Safety & Operational Standards
Calcium bicarbonate doesn’t threaten people with burns, explosions, or toxicity when handled in solution at normal concentrations. Still, operational rules ask workers to prevent splashes from strong acids or bases, which could upset the stability of the ions in water. For large-scale applications—water softening, brewing, cooling towers—equipment maintenance and regular water testing keep calcium bicarbonate at workable levels, so problems with scaling or corrosion stay minimal. Facilities tie safety protocols to overall water chemistry, with clear instructions and records required by local regulations.
Application Area
Check any place where water flows through pipes or sits in tanks, and calcium bicarbonate’s influence shows up. Drinking water systems face challenges with scale, traced directly to the presence of this compound. Municipal engineers invest in softening systems and filter replacements, while homeowners install water softeners to protect appliances and improve taste. Agriculture leans on calcium-rich water to boost crop growth, but farms keep an eye on bicarbonate content because too much throws off soil balance. Beverage makers fine-tune water composition for flavor consistency. Even heritage sites and museums monitor humidity and water ingress because calcium bicarbonate can be a silent architect of decay or preservation.
Research & Development
Research on calcium bicarbonate stretches from the geology of ancient caves to the bleeding edge of water treatment. Universities and engineering firms look at ways to manage scaling using smart chemicals, filtered membranes, and predictive software. Environmental scientists focus on the carbon cycle, tracking how reactions involving calcium bicarbonate influence CO₂ absorption and release in lakes and groundwater systems. Climate researchers measure how much atmospheric CO₂ dissolves into natural waters, knowing chemical reactions with calcium bicarbonate hold a piece of the puzzle around ocean acidification and carbon sequestration strategies.
Toxicity Research
Toxicology reports for calcium bicarbonate produce a collective shrug from both scientists and regulatory agencies. At concentrations found in drinking water, it doesn’t cause acute or chronic health problems. Health departments pay attention to overall mineral balance, as water high in calcium bicarbonate sometimes contributes to kidney stone risk or affects taste. Chronic overconsumption of “hard” water rarely results in serious issues, but regular reviews by public health officials watch for any signs the balance tips in vulnerable individuals. Animal studies confirm a high margin of safety, reinforcing its status as a benign actor in environmental and human health contexts.
Future Prospects
Sustainable water management tackles the challenge of dealing with scaling, taste, and industrial buildup rooted in calcium bicarbonate chemistry. Smart devices now track hardness on a minute-by-minute basis, while eco-friendly treatments replace once-toxic cleaning chemicals. As climate models become more precise, researchers anchor assumptions and predictions in reactions that include calcium and bicarbonate. Decarbonization plans sometimes explore accelerated weathering—where reactions like those forming calcium bicarbonate get harnessed to capture and lock away CO₂. The compound’s subtle power will keep it in the headlines—under different names, in new applications, and always shaping water, stone, crops, and industry.
What is calcium bicarbonate used for?
Everyday Encounters with Calcium Bicarbonate
Tap water in many regions carries more than just H2O. Calcium bicarbonate often rides along, picked up as water flows over limestone or chalk. When water leaves those white spots on your kettle or coffee maker, that’s calcium deposits left behind after the water evaporates. This is hard water—rich in dissolved minerals including calcium bicarbonate. These minerals impact more than household appliances. Scaling from this content clogs pipes, shortens the lifetime of boilers, and adds to maintenance costs for water-dependent equipment. People running commercial laundries or factories with cooling towers know this headache well.
Role in Water Treatment
Municipal water plants remind us of calcium bicarbonate’s double-edged sword. On one hand, it helps buffer pH, stopping water from turning too acidic and corroding pipes. It does this without adding harsh chemicals. On the other, too much contributes to those deposits nobody wants. Plant engineers often turn to softening techniques—lime-soda treatment or ion exchange—to cut down scaling, strike the right balance, and protect their infrastructure.
Calcium Bicarbonate and Health
Besides its chores in plumbing, calcium from water plays a part in our own biology. Drinking water with dissolved calcium makes a difference where diets fall short, especially for bone strength. The World Health Organization recognizes this, hinting there’s a bonus in drinking hard water for calcium and magnesium. In communities where daily diets lack these nutrients, water naturally topping up what’s missing is no small thing. Too much can be a concern for people dealing with kidney stones, but for most, tapping into calcium-rich water closes nutritional gaps.
The Beverage Industry’s Use of Calcium Bicarbonate
Brewers and bottlers study mineral content down to the last milligram. Calcium bicarbonate finds its way into the recipe for mineral waters, seltzers, and even craft beers. In brewing, water chemistry sets up everything from flavor to mouthfeel. Certain styles—think Irish stouts or German lagers—owe part of their character to traditional water sources packed with just the right balance of calcium and bicarbonate. If a city’s tap water won’t do, manufacturers adjust profiles to match classics from around the world.
Environmental Connections
Runoff from farms and city streets changes the minerals in streams and lakes. Calcium bicarbonate helps buffer these water bodies, holding their pH steady so fish and plants survive shifts from acid rain or pollution. Local governments and conservationists keep tabs on water hardness for a reason—it hints at the land and geology nearby, and shapes plans to protect local ecosystems.
Looking for Solutions
Scaling and hard water plague homes and industries. Descaling systems, water softeners, and regular maintenance protect appliances and cut repair bills. Researchers push for new approaches, like eco-friendly softening agents and better monitoring tech. Knowledge helps people manage hardness in water more effectively, whether they run a city water plant or just want their dishwasher to run clean. Educating households about the benefits of certain minerals, yet giving tools to control excess, makes life a lot easier.
Is calcium bicarbonate safe for consumption?
Looking at Calcium Bicarbonate in Our Lives
Calcium bicarbonate probably sounds like something found only in a chemistry lab. A lot of people interact with it more often than they might realize. This naturally occurring compound shows up in drinking water, especially in regions where groundwater travels through limestone. Sparkling water also picks up some extra fizz and “crispness” from dissolved calcium bicarbonate.
Assessing the Safety of Calcium Bicarbonate
Drinking water with dissolved minerals, including calcium bicarbonate, isn’t anything new. Researchers and public health officials have studied “hard water” for decades. Most findings point to the minerals—mainly calcium and magnesium—being safe and, in some cases, even helpful. Big organizations like the World Health Organization have said that drinking water with these minerals does not pose a health risk for the vast majority of people.
On a personal note, I grew up in a rural area where well water tasted different because it was loaded with minerals. Our family never had trouble, but the kettle in the kitchen collected that crusty mineral scale. Tasting those minerals in daily water seemed normal at home, while city water tasted almost flat. Most folks I know in rural places feel the same—if minerals in the water ever caused issues, it wouldn’t be a secret for long.
Calcium bicarbonate especially stands out for people with diets short on calcium. The human body requires calcium to keep teeth strong, help muscles contract, and support hundreds of cellular functions. Unless drinking water contains truly massive quantities, the dissolved form likely just helps meet daily needs.
When Does Calcium Bicarbonate Cause Trouble?
Everything has its limits. Extra calcium in the diet brings benefits, but only to a point. Excess calcium may contribute to health problems, such as kidney stones, in sensitive individuals. The source for most of this risk comes from supplements or highly fortified foods rather than drinking water. Tap water, for the average person, simply doesn’t contain enough calcium bicarbonate to raise red flags.
For those who have special medical conditions, such as chronic kidney disease, any extra minerals matter. These individuals must track what they eat and drink, because their bodies struggle to filter out excess minerals. In these situations, people need guidance from a medical professional. None of this takes away from the fact that for most healthy adults and children, calcium bicarbonate poses little concern in the amounts commonly present.
Solutions and Good Habits
One solution for anyone worried about their water is regular testing. Local health departments usually offer support and information on mineral content in tap water. Homeowners using private wells can get simple tests done for peace of mind. If calcium or other mineral levels ever climb too high, water softening systems help remove excess.
Clear labeling also helps. Beverage companies selling mineral-enriched drinks ought to share the actual content per serving. This supports people making healthy choices, especially if they have unique health needs.
Trusting Facts and Science
Calcium bicarbonate in our water and some drinks works as a reminder: Nature gives our food and beverages plenty of complexity. Most well-established studies and government data show that for the general population, calcium bicarbonate poses no significant risk. People curious about their water or diet can always talk to a registered dietitian or a trusted health care provider. Facts and lived experience show that for almost everyone, calcium bicarbonate is not something to worry about.
How should calcium bicarbonate be stored?
Understanding Calcium Bicarbonate’s Quirks
Calcium bicarbonate acts differently from its cousins in the world of chemicals. It only exists in solution. Many people hunting for a white powder and a bag with “Ca(HCO3)2” stamped on the label run into a wall pretty quickly. You won’t find calcium bicarbonate on a shelf as a dry solid because it falls apart outside of water. In the open air or in a sealed bottle, it tries to return to its more stable versions—calcium carbonate, water, and carbon dioxide.
Why Proper Storage Matters
Anywhere water quality, geology, pools, or lab projects come into play, calcium bicarbonate enters the chat. Its role in buffering water or balancing pH depends on keeping it together. Even small changes—a temperature bump, a drop in pressure, or exposure to air—push it back into calcium carbonate. That transformation can spoil experiments, disrupt water processes, and clog equipment. Avoiding these headaches depends on handling it carefully, with a real focus on storage.
Keeping Solutions Stable: Rules to Live By
Anyone who has mixed up a batch of calcium bicarbonate in a lab or on an industrial scale knows that the solution acts finicky. To keep it stable, it takes a few key moves:
- Store in Sealed Containers: An airtight container makes a difference. Carbon dioxide likes to escape whenever it can. Tight seals keep the CO2 in, slowing down breakdown and preventing calcium carbonate from showing up as sediment at the bottom.
- Control Temperature: Lower temperatures help calcium bicarbonate stay dissolved. Warm conditions invite more gas to escape and speed up decomposition. Cold, dark storage gives the solution the best shot at staying stable, so refrigeration gets the nod if the setting allows.
- Avoid Agitation and Shaking: Stirring and shaking whip CO2 out of solution. The less the container moves, the fewer problems with cloudy solids or reduced effectiveness in your process.
- Check Water Quality: Using distilled or deionized water cuts down on contaminants that might speed up breakdown. Tap water sometimes brings in extra minerals, which trigger faster precipitation and more mess.
Common Traps and Hazards
Many have learned through trial and error that calcium bicarbonate doesn’t like sitting around for weeks. It breaks down quickly compared to other chemicals. Even with perfect storage, expect a shelf life counted in days. Watch for deposits in pipes and equipment, as mineral buildup signals the solution lost its CO2 and reverted to carbonate. This kind of clog can kill water softeners, foul tanks, or throw off test results in the field.
Solutions Backed by Experience
Making only the amount needed, fresh each time, tackles half the struggle. Short storage times mean less risk of wasted solution and less hassle over expired stocks. Glass works better than plastic for containers, as glass holds a seal and doesn’t let CO2 sneak out. Anyone in landscaping, aquatics, or lab work should label containers with mix times and use-by dates, cutting down mistakes and keeping projects humming along.
Reliable storage builds trust in results, whether the job calls for water testing, mineral supplements, or managing pools. Following a few simple steps, based on practical experience and scientific principles, pays off with clearer water, fewer breakdowns, and less wasted money or time.
What are the side effects of taking calcium bicarbonate?
Why People Take Calcium Bicarbonate
Calcium matters to bones, teeth, muscles, and nerves. Some folks turn to things like calcium bicarbonate to help reach their daily intake. Tablets or powder, sometimes found in antacids or water treatments, make it easy to get that boost without gulping down a glass of milk. While calcium helps countless body systems run smoothly, grabbing nutrient pills without pausing to think can come with surprises.
The Ups and Downs: What Happens in Your Body
Swallowing extra calcium may sound like no big deal. In reality, your body has limits. Too much calcium from supplements, especially if you don't need it, can stir up trouble. Stomach upset, gas, and even constipation show up most often. Drinking more fluids or switching brands sometimes eases this, but the stomach still feels the effects before any bones benefit.
Some people feel queasy, get a metallic taste, or report being more thirsty than usual. If the kidneys aren’t firing on all cylinders, these effects can build up. The National Institutes of Health points to what happens over time—high doses can lead to calcium buildup in blood, a condition called hypercalcemia. Signs creep up on you: Nausea, confusion, sore muscles, and kidney stones all tie back to calcium overload. That stone can be as small as a grain of sand and still cause sharp pain and bleeding.
Why It Matters for Certain Groups
Folks with kidney conditions, older adults, or those on certain medications need to watch calcium supplements. Thiazide diuretics—blood pressure meds—slow how quickly you lose calcium in urine, making build-up more likely. A nurse at my local clinic shared stories of patients bouncing in and out of the ER with cramps or palpitations, only to discover calcium was the quiet culprit. Doctors run bloodwork to spot this, but over-the-counter buyers usually miss warning signs.
Story after story comes up in family medicine: People hoping to do right by their bodies but winding up in a worse spot. I remember one patient who doubled up on supplements after reading a magazine article, certain it would strengthen her bones after menopause. By spring, she was showing up with vague aches, only to find her kidneys were starting to struggle.
How to Stay on the Safe Side
Reading nutrition labels and knowing your own eating habits give real answers. Most people get enough calcium eating yogurt, leafy greens, or even canned fish with bones. Supplements should fill gaps, not create new ones. The U.S. Food and Drug Administration points out that 1,000 to 1,200 mg of calcium per day meets most adults’ needs, and going over that mark causes more harm than good.
Doctors and dietitians agree: decide with facts, not fear. A calcium blood test doesn’t cost much and tells you if you even need a supplement. If you’re using an acid-reducing medicine, ask if calcium citrate works better for absorption and causes fewer digestive side effects. Waiting until something feels wrong ignores the quiet way excess calcium causes problems.
Looking Ahead
Preventing side effects from calcium bicarbonate starts with real conversations—between doctors and patients, friends and family, pharmacists and customers. People deserve care tailored to their age, health status, and eating patterns. A flashy label can’t replace medical advice.
Basing choices on evidence from sources like the NIH, Mayo Clinic, and real-world medical stories keeps people healthier in the long run. Respecting the line between enough and too much, asking questions, and focusing on food first keeps bones strong without giving side effects more room to grow.
Can calcium bicarbonate be used to treat calcium deficiency?
Looking at Calcium and the Role of Supplements
Nobody likes feeling weak or dealing with muscle cramps. Many people chalk this up to “not enough calcium.” Doctors and nutritionists do talk about low calcium a lot for good reason. Bones need it. Muscles too. Even the heart depends on the right level of calcium in the blood. So, folks often reach for a supplement. Lately, more people have been asking about calcium bicarbonate.
The Science Behind Calcium Bicarbonate
Calcium bicarbonate isn’t found sitting in a bottle at the local pharmacy. Most people run into it in hard water, where it helps form those chalky stains on faucets and kettles. In nature, water packs some calcium carbonate and carbon dioxide, which can turn into calcium bicarbonate. You can drink mineral water and get some extra calcium this way, but it’s a different story when you want a reliable treatment for deficiency.
What’s Absorbed Matters More Than What’s Consumed
Eating or drinking calcium doesn’t mean the body can actually use it. Our guts need to absorb it. Here, the form of calcium plays the biggest role. Calcium carbonate and calcium citrate are the two most common forms found in supplements, both of which offer reliable results. Calcium bicarbonate only shows up dissolved in water; you don’t see a “calcium bicarbonate” pill because the compound breaks down fast outside this watery environment.
Sometimes people hear about using water with calcium bicarbonate as an easy fix for bone health. But, there’s a catch—bicarbonate breaks down to regular calcium ions and carbon dioxide in the acid-filled stomach. You end up with calcium carbonate again. It’s not some turbo-charged delivery method. So, if the real concern is fixing a calcium shortage in the body, drinking mineral-rich water can help supplement the diet, but it won’t work any better than traditional supplements.
Bioavailability and Absorption Hurdles
The nutrition and medical communities have dug deep into calcium absorption. Calcium citrate stands out for people with less stomach acid. Calcium carbonate, often cheap and widely available, works fine for folks with healthy stomachs and is best taken with food. Calcium from water (even water packed with bicarbonate) just doesn’t add up to as much as the pills.
For people who have a rough time with standard supplements—maybe due to digestive issues or kidney stones—getting enough from water alone just doesn’t fit the bill. Milk, leafy greens, and certain fortified foods do a better job. So does sticking to what’s proven: supplements with verified absorption rates.
Consider the Risks and Talk with Pros
Far too often, people jump on trends they read online or hear from friends, and skip checking with a medical professional. Too much calcium can lead to kidney stones. Incorrect supplementation can mask other serious issues. In severe calcium deficiency, such as hypocalcemia from parathyroid trouble or certain medicines, a doctor looks at prescription therapies, not just over-the-counter pills or water.
Solutions Go Beyond a Single Ingredient
Drinking mineral water rich in calcium can help boost daily intake, but it shouldn’t become the only plan for treating deficiency. Most studies back up a food-first approach. Real foods carry more nutrients, often help balance absorption, and lower the odds of going overboard. For people who need more, trusted supplements with calcium carbonate or citrate carry the strongest track record. Getting guidance from a nutritionist or doctor locks in the best path and helps avoid risky mistakes. Your bones and your muscles deserve more than a guess.
| Names | |
| Preferred IUPAC name | calcium hydrogencarbonate |
| Other names |
Carbonic acid calcium salt Calcium hydrogen carbonate |
| Pronunciation | /ˈkalsi.əm baɪˈkɑː.bə.neɪt/ |
| Preferred IUPAC name | calcium hydrogencarbonate |
| Other names |
Calcium hydrogen carbonate Bicarbonate of lime |
| Pronunciation | /ˈkæl.si.əm baɪˈkɑː.bə.neɪt/ |
| Identifiers | |
| CAS Number | 5743-26-0 |
| 3D model (JSmol) | `JSmol` model string for **Calcium Bicarbonate**: ``` Ca2+.[HCO3-].[HCO3-] ``` |
| Beilstein Reference | 3589695 |
| ChEBI | CHEBI:91747 |
| ChEMBL | CHEMBL1201731 |
| ChemSpider | 22688 |
| DrugBank | DB11093 |
| ECHA InfoCard | 200-578-6 |
| EC Number | EC 238-358-9 |
| Gmelin Reference | 15171 |
| KEGG | C05443 |
| MeSH | D002121 |
| PubChem CID | 64798 |
| RTECS number | EW2625000 |
| UNII | WYQ29042BH |
| UN number | Not assigned |
| CAS Number | 3983-19-5 |
| Beilstein Reference | 4150825 |
| ChEBI | CHEBI:91753 |
| ChEMBL | CHEMBL1257075 |
| ChemSpider | 22701622 |
| DrugBank | DB11338 |
| ECHA InfoCard | ECHA InfoCard: 03-03-01-01703-53 |
| EC Number | 208-167-3 |
| Gmelin Reference | 66346 |
| KEGG | C05933 |
| MeSH | D001139 |
| PubChem CID | 3084063 |
| RTECS number | FF9350000 |
| UNII | 91U12N1DT8 |
| UN number | UN1846 |
| CompTox Dashboard (EPA) | urn:uuid:30e47a80-631d-4fd7-b839-ffb9b82bc5ec |
| Properties | |
| Chemical formula | Ca(HCO3)2 |
| Molar mass | 162.114 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 2.2 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.3 |
| Acidity (pKa) | 15.2 |
| Basicity (pKb) | 9.58 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Dipole moment | 0 D |
| Chemical formula | Ca(HCO3)2 |
| Molar mass | 162.114 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 2.2 g/cm³ |
| Solubility in water | Slightly soluble |
| Acidity (pKa) | 10.4 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | Ca(HCO3)2: 199.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −213.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -234.6 kJ/mol |
| Std molar entropy (S⦵298) | (-) S⦵298 = - |
| Std enthalpy of formation (ΔfH⦵298) | -1136 kJ/mol |
| Hazards | |
| Main hazards | No significant hazards. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. |
| NFPA 704 (fire diamond) | 1-0-0 |
| PEL (Permissible) | PEL for Calcium Bicarbonate: Not established |
| REL (Recommended) | 200 mg |
| IDLH (Immediate danger) | Not listed |
| Main hazards | May decompose to emit irritating and toxic fumes of carbon dioxide and calcium oxide. |
| GHS labelling | GHS labelling for Calcium Bicarbonate: "Not a hazardous substance or mixture according to the Globally Harmonized System (GHS) |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS) |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 600 mg |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
Calcium carbonate Calcium hydroxide Calcium acetate Sodium bicarbonate Magnesium bicarbonate |
| Related compounds |
Calcium carbonate Calcium hydroxide Calcium acetate Magnesium bicarbonate Sodium bicarbonate |
| Pharmacology | |
| ATC code | A12AA04 |

