Calcium Acetate: A Comprehensive Look at Its Past, Present, and Future
Historical Development
Calcium acetate has served practical roles long before most people cared about its chemical formula. Vinegar poured over burnt lime once offered a crude method for making it centuries ago, with pharmacists and tanners finding its uses without today’s precision. Over time, makers refined the process, shifting from vinegar and burnt shells to controlled reactions in factories. The drive to use leftover materials from food processing and the iron industry shaped commercial production. As medical science started to see the danger of phosphate buildup in patients with kidney issues, calcium acetate took on a new role in the pharmacy, finding its place on shelves thanks to studies stretching back to the 1960s. The once-humble salt has gained significance as research and industry caught up with its hidden promise.
Product Overview
People often spot calcium acetate as either a white powder or a colorless crystal, usually odorless and easy to settle into mixtures. Packagers deal it in bags or drums, shipping it worldwide for industries ranging from pharmaceuticals to water treatment. The product comes labeled for specific uses: as a food additive (E263), a medicine for binding extra phosphate, or a chemical reagent. Price, grade, and regulatory compliance drive the market. Food scientists and pharmacists pay close attention to these designations, as only some forms meet medical standards or food purity requirements. Regulatory bodies like the FDA and EFSA keep close tabs, pushing producers to maintain a standard reputation.
Physical & Chemical Properties
This compound doesn’t surprise much in the lab. It forms white, hygroscopic crystals, dissolves well in water, not so much in alcohol, and won’t stand up to open air for long without pulling in moisture. The molecular weight hits around 158, and it breaks apart into calcium and acetate ions in solution. No sour bite, no scent, just a basic salt that holds its shape until asked to react. Heat it high enough, and calcium acetate leaves acetic acid and calcium oxide behind. The mix keeps steady across a range of pH values, giving it flexibility for pharmaceutical suspensions, dialysis solutions, even bakery recipes.
Technical Specifications & Labeling
Producers must stick to purity levels, particle sizes, and limits on impurities like lead or arsenic when selling calcium acetate. Labels on pharmaceutical grades stress batch numbers, expiry dates, and certifications tracing back to manufacturing plants. For food or industrial uses, labels focus more on composition and moisture content. Product certificates line up with international standards like USP, Ph. Eur., and JP, holding up under audits and customer reviews. Customers looking for kosher or halal compliance find these marks on packaging, thanks to rigorous checks at every processing stage.
Preparation Method
Production begins with common sources: calcium carbonate (from limestone, marble, or shells) reacts with acetic acid. Commercial batches skip slow, traditional methods and rely on reactors maintaining temperature control, churning out crystalline calcium acetate under watchful eyes. Waste management steps collect carbon dioxide and recover heat, keeping environmental impact in check. After reaction, filtration screens out insoluble bits, and evaporators bring the solution to a solid state, ready for grinding and packaging. Each method tweaks conditions for either food, pharmaceutical, or technical grades, with purification steps or additives added as needed.
Chemical Reactions & Modifications
Beyond its base use, calcium acetate brings life to several reactions. Mixing with sodium carbonate creates calcium carbonate, helping soften water. In the lab, heating calcium acetate breaks it down to create acetone, a trick used in organic synthesis since the days of early chemistry. Reacting with strong acids frees up acetic acid, valuable for both flavor and cleaning industries. Technologists experiment with coating granules, mixing with stabilizers, or pairing with other binders, exploring new ways to broaden its reach, especially for novel pharmaceutical delivery forms and slow-release fertilizers.
Synonyms & Product Names
Throughout the global market, calcium acetate shows up under many monikers. In pharmaceutical catalogs, it’s listed as PhosLo or Calcium ethanoate. Food chemists note it as E263, while old-school texts call it calcii acetas. Some suppliers tag it with CAS number 62-54-4. In practice, these names signal purity grade, end-use, and sometimes the region, but the molecule stays the same, serving as a familiar building block across continents.
Safety & Operational Standards
Workspaces handling calcium acetate stick to clear-cut protocols. While not considered highly toxic, dust may irritate airways or skin, and eye contact causes discomfort. Workers rely on gloves, masks, and local exhaust systems, particularly when packaging or blending powder in bulk. Storage keeps the product sealed and dry, since moisture can clump or degrade quality. Regulations in the US, EU, and Asia spell out acceptable exposure and purity limits, with routine testing to catch cross-contamination with heavy metals or biological material. Disposal laws steer companies away from tossing leftovers into the environment, pushing them to treat or repurpose waste if possible.
Application Area
Calcium acetate shines in many places. Hospitals prescribe it to support kidney patients by trapping phosphates in food, easing the workload on damaged kidneys. Food companies toss it in as a preservative, holding back spoilage in baked goods while lowering acidity in certain pickled items. Sewer control crews use it to keep hydrogen sulfide from forming in pipes, cutting down on odors and corrosion. Water treatment plants bank on it to soften water, and some industrial firms employ it as a setting accelerator in concrete. Chemists see it as a handy reactant, a bridge to acetone and other compounds.
Research & Development
Researchers dig deeper year after year, building on calcium acetate’s reliability. New forms like extended-release tablets could help patients keep phosphate in check with fewer doses. Food technologists test blends with other preservatives, slicing costs or enhancing shelf-life for gluten-free bread. Civil engineers look for greener setting agents in construction, hoping calcium acetate fits the bill. In labs, attention turns to purer grades for next-gen pharmaceutical processes or as a base for new organic syntheses. Industry and academia often swap notes, driving grants and pilot projects that blend health, food, and chemical manufacturing interests.
Toxicity Research
Safety research keeps a close eye on calcium acetate, especially with its widespread use among vulnerable groups like dialysis patients. Controlled trials track side effects, mostly gastrointestinal like nausea or constipation at high doses. Reports from long-term therapies continue, noting rare but serious risks such as hypercalcemia if supplements stack up in the system. Regulators want more data on combined effects with other drugs or underlying medical conditions. Food industry doses don’t come close to these risks, but agencies watch new findings, keeping stricter upper limits just in case. Animal studies and environmental assessments feed into global regulatory updates every few years.
Future Prospects
Farming practices shift and water shortages loom, so demand for eco-friendly soil conditioners and water softeners will likely grow. In healthcare, personalized medicine pushes for precise phosphate binders—calcium acetate stands to hold ground if reformulated with fewer side effects or combined with new drugs. Green chemistry labs look to repurpose or recycle byproducts, making production less wasteful. Calls for clean-label food additives offer steady business, so suppliers invest in traceability programs and cleaner manufacturing. As research links new health and industrial uses to this reliable salt, it keeps its foothold, evolving along with technology and rising sustainability expectations.
What is Calcium Acetate used for?
In the Pharmacy: Helping Patients with Kidney Disease
Doctors see a lot of patients struggling with kidney failure. One thing that comes up a lot is high phosphate levels in the blood. Kidneys usually handle that, but when they can't do their job, phosphate piles up. High phosphate can weaken bones and harm blood vessels. Calcium acetate steps in as a phosphate binder. Patients take it with meals, and it grabs onto phosphate in food, so less makes its way into the bloodstream. Most people don't realize it, but this simple pill can delay some pretty nasty complications that come from end-stage kidney problems. Several major studies have shown that calcium acetate works safely over long periods and doesn’t load the body with too much calcium if it’s monitored closely.
On the Table: A Role in Food Processing
Processed foods last longer and stay tasting fresh with a little help from certain ingredients. Calcium acetate acts as a preservative in some settings. Small bakeries count on it in bread, where it keeps things from molding before the loaf ever makes it to the dinner table. Big producers of sweets and jellies use it too. I’ve always read ingredient lists, and you don’t see it everywhere like salt, but behind the scenes, it keeps products safe for longer, especially in humid climates.
On the Streets: Deicing and Dust Control
Walk down the street after a snowstorm and you’ll notice rock salt everywhere—it keeps roads safe but trashes pipes, car parts, and river water. Calcium acetate, when applied as a road treatment, melts ice just as well without causing so much rust or environmental damage. City crews deploy it on sensitive roads and near waterways. Modern communities look for greener ways to clear snow, and this option keeps showing up in more local government contracts.
Behind the Scenes: From Lab to Daily Life
Chemistry classrooms often use calcium acetate for more than lectures. Mix it with ethanol and you get “solid alcohol,” which burns cleaner than regular fuel and finds use in emergency heat packs or portable cookers. It sounds like a simple trick, but in search and rescue work, or in military kits, this blend can offer quick warmth or basic hot food without dangerous fumes. Growing up in a camp-heavy family, I’ve seen these fuel packs save summer trips and help out during winter storms.
Choosing the Right Use: What’s Best for Each Need?
It’s easy to overlook an ingredient like this because it never hogs the spotlight, but the ripple effects show up in medicine, food, and public safety. For kidney patients, monitored use makes all the difference by preventing long-term complications of too much phosphate. Food regulators recommend it in limits to keep the diet safe overall. In winter, cities keep water and soil quality on their minds and push for options like calcium acetate to reduce the environmental headache that comes with road salting.
A Place in the Modern World
People look for alternatives that keep their families and communities safe and healthy. Calcium acetate stands out as one of those unsung helpers—quietly important on the dinner plate, on icy roads, and in the pharmacy. It stands as a reminder that small tweaks, even in basic chemicals, often deliver health and environmental payoffs that ripple out into everyday life.
How should I take Calcium Acetate?
Why Doctors Recommend Calcium Acetate
People battling kidney problems often hear their doctor bring up phosphate binders, and calcium acetate sits near the top of that list. I remember meeting a neighbor who had high phosphate because of failing kidneys and couldn’t touch many foods he loved. Instead, he had a prescription for little white pills: calcium acetate, taken before meals. Each meal brought a tough choice—eat what he wanted, or stick to strict rules set by a condition he never asked for.
How Calcium Acetate Works in Your Body
After meals, food breaks down, releasing phosphate. Your kidneys help clear extra phosphate, but once kidneys lose strength, phosphate levels go up fast. Too much phosphate can cause bones to weaken and bring on serious heart problems. Taking calcium acetate with food traps phosphate in your gut before your body can soak it up. With fewer phosphate molecules moving into your blood, risks drop.
Best Way to Use Calcium Acetate
Doctors write clear instructions: take calcium acetate with each meal or snack—never on an empty stomach. Skipping this timing wastes the chance to block phosphate. Swallow the tablets whole, using a good drink of water. Some folks find themselves swallowing a handful at a time, which can feel like a headache, but breaking the routine brings trouble faster than most expect.
Anyone with difficulty swallowing pills should ask their pharmacist if splitting or crushing pills is safe, as some brands don’t offer that option. Liquid forms exist, but always match your doctor’s advice to the letter.
What to Watch For
I’ve seen people worry when cramps or constipation pop up after starting these tablets. Too much calcium in the blood brings its own set of risks, leading to confusion, fatigue, or irregular heartbeat. Health experts always suggest bloodwork to see if calcium or phosphate levels leave the safe range. If a person feels sick or notices swollen ankles, trouble breathing, or tummy pains, a call to the clinic beats waiting things out.
Simple Strategies to Stay on Track
People easily lose track of doses when life gets busy. Pulling out a pill case and setting alarms on the phone help a lot. Family members who understand the importance can act as daily support. Clinics sometimes run classes, teaching patients how food choices affect their results, and nobody should feel embarrassed to ask questions.
Talking to Your Team
Discussing all medications and supplements matters because other pills can throw calcium levels off or cause kidney stones. Grapefruit juice, antacids, or multivitamins can tip the scale. Doctors often check up every few weeks at first, adjusting how many tablets people take depending on their blood tests. Skipping tests means risking harm nobody wants.
Toward Better Health
Taking calcium acetate becomes part of daily life for some people with serious kidney troubles. Admitting the routine isn’t always easy makes a difference—personal experience shows me most folks only stick with tough medication plans when they truly understand what’s at stake. Looking out for symptoms, talking openly with doctors, and sticking to a reliable routine offer the best shot at keeping health from slipping away.
What are the side effects of Calcium Acetate?
Understanding Calcium Acetate’s Role
Calcium acetate pops up in the world of medicine mostly for folks with chronic kidney disease. It helps lower phosphate levels in the blood, since damaged kidneys can’t filter things like they used to. Doctors often prescribe this if someone’s phosphate creeps too high, because extra phosphate in the body can pull calcium from the bones and cause hardening of tissues and vessels. Lowering phosphate keeps things healthier for the heart and bones.
Common Side Effects—What People Actually Notice
Stomach issues lead the pack. I’ve talked to patients who say their stomach feels off after a few doses: nausea, bloating, sometimes just a general discomfort. Diarrhea and constipation also make the rounds. One of my old neighbors used to try and time his pills with meals, but he swore that some foods made the side effects worse. Sometimes cramps set in, or people complain about gassiness.
These stomach troubles aren’t great, but they’re usually not dangerous. Still, they can chip away at someone’s willingness to stick with the medicine. I remember folks just giving up and risking the phosphate instead, so the discomfort can have bigger medical consequences over time.
Worrying Signs—When the Risks Grow
Too much calcium poses a real problem over the long haul. If someone takes a lot of calcium acetate and doesn’t get routine blood work, they can wind up with high levels of calcium—hypercalcemia. The first clues don’t always shout. Maybe a person feels tired, confused, loses their appetite, has more headaches. If the calcium keeps climbing, it threatens kidneys, can throw the heart rhythm out of rhythm, and, in rare cases, leads to a kidney stone.
People on calcium-based binders need blood tests. This isn’t just to check how much phosphate drops; it’s to keep an eye on calcium creeping too high. If a doctor spots calcium above range, they may cut back the dose or suggest a different phosphate binder.
Long-Term Questions and Solutions
Doctors and patients keep talking about whether calcium acetate is best for the long haul. Questions stick around, because loading the body with extra calcium isn’t a long-term answer for every patient. A few researchers have brought up that too much calcium can land in soft tissues, leading to calcification—like extra cement in the wrong spaces.
Diet can help manage phosphate but doesn’t always do enough, especially for dialysis patients. Regular blood work matters as much as the medicine. People who feel especially bad on calcium acetate might ask their doctor about non-calcium phosphate binders like sevelamer. Those other binders steer clear of increasing calcium while lowering phosphate.
Working Together With Doctor and Patient
Nobody likes to feel sick from the medicine that’s supposed to keep them healthy. Open conversations matter most. If a patient skips doses because of an upset stomach or just feels poorly, the care team needs to know. Pharmacists often catch things, suggesting splitting up the dose or adjusting meal timing. Families get involved, too, especially when symptoms aren’t easy to notice—confusion, weakness, or appetite changes. The key with calcium acetate is to keep the conversation going and make changes before things get out of hand.
Can I take Calcium Acetate with other medications?
Understanding How Calcium Acetate Works
Calcium acetate is usually prescribed for folks dealing with kidney disease, especially those on dialysis. It keeps phosphate levels in check. High phosphate shows up as bone troubles and causes itchiness, and sometimes the damage runs deeper. So keeping phosphate down gives the body some relief.
Mixing Calcium Acetate With Other Medications
Calcium acetate doesn’t always play nice with other medications. It can cling to certain drugs in the gut and block their absorption. I’ve seen patients struggle with warfarin, thyroid pills, even some antibiotics when they swallow them alongside binders. Imagine planning your whole pill schedule around something as simple as calcium tablets.
Consider levothyroxine, a common pill for thyroid disease. Swallow it with calcium acetate, and the body might not absorb enough. Suddenly, a person who was feeling fine feels sluggish. Doctors regularly see this and often end up chasing lab results, unsure if the dose is too low or if the pill schedule just needs some tweaking.
Antibiotics like ciprofloxacin and tetracycline run into the same problem. Calcium binds to these drugs, forming insoluble chunks, throwing off their effect. People with serious infections can’t afford to miss a dose or get only half of it working in the body. It might seem like a minor hitch, but skipping antibiotics or reducing their power risks a bigger, costlier health problem.
Timing Makes a Difference
Doctors often recommend spacing out doses of calcium acetate and other medications by at least two hours. This buffer keeps each pill from interfering with the others. In my experience, folks coming into a kidney clinic already have a table full of pill bottles. Asking them to organize timing can feel overwhelming, especially if they need to take meals into account.
Still, people who set reminders or build a daily system tend to manage better. I’ve watched patients pull out pill sorters with sticky notes for each hour. This extra planning doesn’t just help drug absorption—a regular schedule boosts confidence and keeps trust in the healthcare partnership.
Talking With Pharmacists and Doctors
A lot of these medication clashes happen quietly. Many people don’t realize that something as basic as a calcium supplement could blunt the effect of heart, thyroid, or seizure medicines. Pharmacists can spot many of these dangers right away, often quicker than anyone else on the care team.
Having a complete list of all pills—prescription, over-the-counter, even herbal supplements—becomes more than a checklist. It’s essential for stopping bad interactions before they start. Not everyone knows that certain multivitamins, antacids, or iron pills can muck up the works even further, so sharing the whole story makes a difference.
Solutions to Avoid Medication Mix-Ups
Managing complex pill schedules works best with teamwork. Digital reminders help, as does old-fashioned written schedules. Pharmacists show patients how to separate doses and which drugs can be taken safely together. Investing time upfront saves frustration, phone calls, and risks down the line.
People deserve to feel that their health isn’t just a list of chores. Knowing about the risks of combining calcium acetate with certain pills brings back a bit of control. Having real conversations with the care team—and asking the questions that feel silly—often prove the most useful.
Who should not take Calcium Acetate?
Understanding Calcium Acetate and Its Purpose
Calcium acetate shows up on prescription pads for people dealing with kidney disease, mostly for those who must keep a steady grip on their phosphate levels. It helps bind phosphate in the gut and keeps blood from getting overloaded. It’s easy to see why doctors reach for it, since too much phosphate builds up fast when kidneys start lagging behind. Not everyone’s a match for this medication, though, and pushing through with it in the wrong body can cause harm that outweighs benefits.
High Blood Calcium: Trouble Hiding in Plain Sight
Folks with already high calcium levels find themselves in the danger zone if they start popping calcium acetate. Extra calcium, especially when kidneys struggle, tends to roll downhill and pool in places where it shouldn’t. Blood vessels can harden, bones lose strength, and confusion sets in. Studies out of places like the National Institutes of Health link high blood calcium to heart rhythm changes and kidney stones. In my years working with patients, I’ve seen how an innocent pill turns into a nightmare just because nobody checked that lab number first.
Kidney Stones: History Matters
Anyone who has dealt with kidney stones remembers the pain all too well. Calcium-based stones land people in the ER, and calcium acetate can raise the risk for anyone already prone to them. Doctors often look back at medical histories, and for good reason — research papers in nephrology journals mention increased recurrence among calcium stone-formers who take calcium-rich binders. Drinking more water, cutting back on salt, and finding alternative phosphate binders make life easier than rolling the dice on another round of stones.
Medications That Clash with Calcium Acetate
Mixing calcium acetate with certain antibiotics can turn an infection into a stubborn foe. Tetracyclines and quinolones get tied up by calcium and never quite do their job. Digoxin, a heart medicine, gets riskier in the presence of higher calcium — causing more side effects. This isn’t just theory; a pharmacist once pointed out, in a case conference I attended, how calcium acetate wrecked the timing of a gut medication, and the patient ended up back in the hospital. People who take large numbers of regular meds should talk it through with their doctor or pharmacist and map out a strategy together.
Parathyroid Problems and Vitamin D Risks
Health conditions like parathyroid disease send calcium levels skyrocketing all by themselves. If someone has untreated hyperparathyroidism or overactive thyroid, calcium acetate steers things further off course. Add in high doses of vitamin D, which pushes up calcium in another way, and trouble comes quickly. Backed by the evidence in clinical guidelines and textbooks, doctors often avoid calcium acetate if labs show high calcium or complicated endocrine issues. A quick blood draw and honest conversation save a lot of trouble.
Smart Practices for Safer Choices
The easiest way to avoid disaster starts with full disclosure: letting the doctor know about all the pills, vitamins, and health problems on the list. Routine lab work uncovers hidden dangers before they grow. Alternatives such as sevelamer or lanthanum carbonate give options to those at risk. Advocates like the American Kidney Fund recommend patient education along with careful medication management, so nobody ends up trading one health problem for another.
Looking Ahead: Staying Informed
Every new prescription deserves respect, and that means reading up, asking questions, and giving a full medical history. As both a clinician and a patient, I see the best outcomes in people who keep the conversation going with their healthcare teams. With enough attention and teamwork, calcium acetate serves its intended purpose for some, while others find better solutions fitting their bodies and lives.
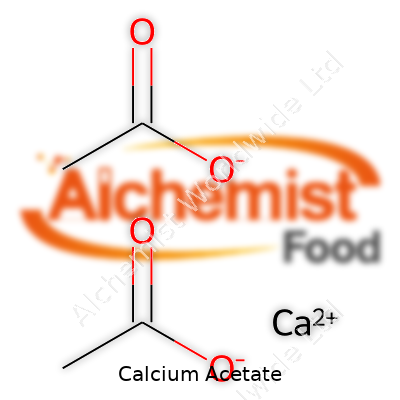
| Names | |
| Preferred IUPAC name | Calcium diacetate |
| Other names |
Calcium diacetate Acetic acid, calcium salt Calcium ethanoate |
| Pronunciation | /ˈkæl.si.əm ˈæs.ɪ.teɪt/ |
| Preferred IUPAC name | Calcium diacetate |
| Other names |
Calcium ethanoate Calcium diacetate Acetate of lime |
| Pronunciation | /ˈkæl.si.əm ˈæs.ɪ.teɪt/ |
| Identifiers | |
| CAS Number | 62-54-4 |
| Beilstein Reference | 391873 |
| ChEBI | CHEBI:31344 |
| ChEMBL | CHEMBL1200997 |
| ChemSpider | 53104 |
| DrugBank | DB00366 |
| ECHA InfoCard | 03cba0c7-3bf2-4561-82a9-cdf477a1d975 |
| EC Number | 204-814-9 |
| Gmelin Reference | 85230 |
| KEGG | C01866 |
| MeSH | D000079 |
| PubChem CID | 305 |
| RTECS number | AF7870000 |
| UNII | 57M87603N8 |
| UN number | UN1748 |
| CAS Number | 62-54-4 |
| Beilstein Reference | 3908587 |
| ChEBI | CHEBI:31344 |
| ChEMBL | CHEMBL1200291 |
| ChemSpider | 5281 |
| DrugBank | DB00364 |
| ECHA InfoCard | 03bab04b-d340-419b-9461-409236cbe7e0 |
| EC Number | 208-140-2 |
| Gmelin Reference | 37984 |
| KEGG | C00628 |
| MeSH | D017379 |
| PubChem CID | 3057 |
| RTECS number | AF7875000 |
| UNII | 9ZV1UI369E |
| UN number | 1327 |
| CompTox Dashboard (EPA) | DTXSID4040606 |
| Properties | |
| Chemical formula | Ca(C2H3O2)2 |
| Molar mass | 158.17 g/mol |
| Appearance | White, hygroscopic powder |
| Odor | Odorless |
| Density | 2.16 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.39 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 12.6 |
| Basicity (pKb) | 15.3 |
| Magnetic susceptibility (χ) | -4.8e-6 |
| Refractive index (nD) | 1.422 |
| Viscosity | 5 - 15 mPa.s (10% aq., 25°C) |
| Dipole moment | 0 D |
| Chemical formula | Ca(C2H3O2)2 |
| Molar mass | 158.17 g/mol |
| Appearance | White, hygroscopic powder |
| Odor | Odorless |
| Density | 1.509 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.39 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 12.7 |
| Basicity (pKb) | 5.3 |
| Magnetic susceptibility (χ) | −29×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.422 |
| Viscosity | 5 mPa·s (20°C, 2% solution) |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 157.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1158.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1134.9 kJ/mol |
| Std molar entropy (S⦵298) | 157.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1016.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1134.6 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A12AA04 |
| ATC code | A12AA09 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, GHS Hazard Statement: H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P305+P351+P338, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Lower: 3.2% Upper: 16.0% |
| Lethal dose or concentration | LD50 Oral Rat 4,290 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 4,260 mg/kg |
| NIOSH | N0404 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Calcium Acetate: Not established |
| REL (Recommended) | 0.2 mg/m³ |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. May cause skin irritation. Harmful if swallowed. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H319 Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Wash hands thoroughly after handling. |
| NFPA 704 (fire diamond) | Calcium Acetate NFPA 704: 1-0-0 |
| Autoignition temperature | > 580 °C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Oral Rat 4,280 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,340 mg/kg (rat, oral) |
| NIOSH | NM 0182128 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Calcium Acetate: Not established |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Calcium carbonate Calcium chloride Acetic acid Sodium acetate |
| Related compounds |
Calcium citrate Calcium carbonate Calcium chloride Magnesium acetate Sodium acetate |

