Butyric Acid: Development, Properties, and Future Directions
Historical Development
Butyric acid carries a long story, stretching back to its discovery in rancid butter by Michel Chevreul in 1814. This organic acid introduced chemists to a distinctive, unpleasant odor and a wide range of uses early on. Chemists have traced the acid’s presence across natural products for generations, with fermentation processes and dairy products revealing its role in everyday life. Over time, manufacturers found ways to produce it on an industrial scale through chemical synthesis and microbial fermentation. The move away from natural extraction allowed for greater purity, stable supply, and cost efficiency—key reasons the chemical gained attention in pharmaceuticals, food, and animal feed sectors. Butyric acid holds a place in both the history of organic chemistry and in the advance of modern industry.
Product Overview
Butyric acid, known to most as butanoic acid, appears as a colorless liquid under ambient conditions and releases a sharp, foul smell reminiscent of spoiled dairy. This strong odor often draws immediate reactions in the lab or processing plant, yet its characteristics allow for significant commercial application. Producers typically manage large volumes of butyric acid to serve as an additive in animal feeds and preservatives in foodstuffs. The acid’s sodium and calcium salts enter medicines, fragrances, and flavor enhancers. Many users rely on the acid’s ability to support gut health in livestock, making it a foundational ingredient in feed blends. It balances practicality with natural origins.
Physical & Chemical Properties
The acid’s chemical formula sits at C4H8O2, and its structure—a simple four-carbon chain with a terminal carboxylic acid group—gives it both reactivity and volatility. It boils at 163.5 °C, with a melting point sitting around -7.9 °C. Solubility in water counts as high, making it easy to blend with other polar substances. These physical traits, with a density near 0.958 g/cm³ and a notable miscibility with organic solvents, let producers easily integrate it into many chemical reactions. The acid’s presence shows up in esters, those fruity compounds found in perfumes and flavorings, created through well-known reactions with alcohols.
Technical Specifications & Labeling
In commercial channels, butyric acid arrives with a purity standard often above 98%. Labels on drums or containers reflect precise chemical identification: butanoic acid, CAS number 107-92-6, molecular weight 88.11 g/mol. Quality control teams test batches for water content, acidity, and trace metals. Shipping regulations draw attention to the acid’s corrosive nature, and the label must show hazard pictograms and UN numbers, calling out the need for careful handling. Packaging uses either high-density polyethylene or stainless steel to prevent corrosion and leaks.
Preparation Method
Manufacturers produce butyric acid through both fermentation and chemical synthesis. Fermentation takes natural sugars—often sourced from corn or molasses—and lets Clostridium tyrobutyricum bacteria convert these into butyric acid under anaerobic conditions. This bio-based route offers sustainability and ties the product to renewable resources. Chemical methods, by contrast, involve oxidation of butyraldehyde, itself derived from propylene through oxo synthesis. Producers run this process under controlled temperature and pressure for consistency and yield. Both methods provide high-purity acids, but fermentation appeals more to industries with green ambitions or strict residue tolerances.
Chemical Reactions & Modifications
Butyric acid reacts with alcohols in the presence of acidic catalysts to form butyrate esters—valuable intermediates for perfumes and food flavors. Conversion to its salts—sodium butyrate and calcium butyrate—matters in animal nutrition, since these forms carry improved palatability and stability. Dehydrogenation can convert butyric acid to crotonic acid, adding value in polymer syntheses. Beyond these core reactions, chemists look at halogenation, amidation, and redox steps to build a ladder of value-added derivatives, each with specialized roles in health, coatings, and plastics.
Synonyms & Product Names
Butyric acid goes by many aliases in markets and technical documentation. Names like butanoic acid, n-butyric acid, and propylformic acid crop up across safety data sheets and spec tables. Trade names and brand names—frequently tied to suppliers in Europe, China, and the US—often keep the base word “butyrate” or “butyric” visible for customer clarity. Alternate chemical references like UN 2820 signal transport hazards instead of composition.
Safety & Operational Standards
Anyone handling butyric acid confronts a pungent irritant. Gloves, goggles, and adequate ventilation matter in storage and transfer. The acid causes burns on skin or eyes. Fumes irritate the nose and lungs, so fume hoods and spill kits become routine parts of the workspace. Facilities follow Occupational Safety and Health Administration (OSHA) guidance and international standards set by the Globally Harmonized System (GHS). Training on storage temperature, emergency procedures for spills, and hazardous-waste disposal support safe plant operations. Investing in proper material compatibility for pipes and tanks stops corrosion and leaks before they can cause damage.
Application Area
Animal nutrition absorbs one of the largest shares, with butyric acid and its salts added to poultry and swine feed. Butyrate’s impact on gut health, growth rates, and feed efficiency pulls strong demand from farm operators. In the flavor and fragrance industries, butyric esters find use as bread, cheese, or fruit flavor surrogates, masking unwanted notes and building new aroma profiles. Pharmaceuticals value calcium and sodium butyrate for potential anti-inflammatory and gene-regulation properties, feeding a steady pipeline of gut health studies. Plastics, coatings, and cigarette industry players all find niches where this short-chain fatty acid modifies product texture or flavor.
Research & Development
Research groups in food science and biochemistry push frontiers on butyric acid’s impact on gut barrier function, the microbiome, and even cancer therapy. R&D teams investigate bio-fermentation strains that deliver greater yield and efficiency, eyeing waste valorization to reduce production costs. Application research focuses on fine-tuning release in animal feeds with microencapsulation, expanding reach into aquaculture and companion animal markets. In pharma, trials using butyrate derivatives measure outcomes for inflammatory bowel disease, neurological disorders, and rare genetic conditions.
Toxicity Research
Toxicologists have run extensive profiles on the acid. Acute oral exposure causes irritation and mild toxicity, while inhalation can provoke respiratory symptoms. Butyric acid shows low systemic toxicity at typical workplace levels, but direct skin or mucous membrane contact leads to burns. Chronic exposure in industrial settings remains rare but calls for ongoing monitoring and proper safety parameters—something regulatory agencies emphasize in training materials and workplace standards. Environmental studies have focused on biodegradability and aquatic toxicity, finding the compound degrades rapidly and poses modest threats at accidental-release levels.
Future Prospects
Sustainable production and new medical applications steer most of the interest in butyric acid’s future. Companies now test bioreactor designs that tap agricultural waste for carbon sources, seeing a double win in waste reduction and improved margins. Feed additive suppliers work to maximize the acid’s benefits for animal health, tracing performance in trials to find new recipes for gut health support that answer both productivity and animal welfare mandates. In human health, the gut-brain axis has drawn even more scientific attention, with butyrate’s signaling roles tying into therapies for mood, memory, and chronic inflammation. Regulations on food quality, flavorings, and biosourced chemicals will shape market growth, but ongoing research and supply innovation keep butyric acid relevant across fields.
What is butyric acid used for?
Old Cheese or Essential Ingredient?
Most people have never heard of butyric acid, but chances are you’ve crossed paths with it already. The unmistakable smell of rancid butter, sweaty feet, or stinky cheese—that’s butyric acid at work. Folk might hold their nose, yet this stuff does more than ruin a room. It stands at the crossroads of food science, animal nutrition, and even health supplements.
Boosting Animal Growth and Gut Health
Visit any livestock farm, and there’s a fair chance butyric acid is part of the picture. It finds its way into feed as a supplement. Studies done by the American Society of Animal Science show pigs and poultry given butyric acid grow faster and digest their food better. Animals get sick less, saving farmers headaches and money. Why does it work? Butyric acid feeds cells lining the gut, helping them grow stronger. This makes animals less prone to disease. It also acts against harmful bacteria, which keeps antibiotic use in check. That’s a win for everyone—farmers, consumers, and public health.
Setting Flavors in Motion
Head to the kitchen, and butyric acid pops up again. Food scientists use it to imitate butter and cheese flavors in packaged goods—from snacks to candies to sauces. Take a whiff of artificial butter flavor on popcorn. That charm doesn’t come from dairy cows. Cheaper, more stable flavors can cut corners for large companies, but butyric acid doesn’t just trick the tastebuds. In small doses, it can wake up the senses with creaminess or sharpness without overpowering. Careful handling is key; use too much, and you cross from gourmet to garbage can real quick.
Gut Microbes and Health Trends
Lately, butyric acid has grown popular beyond farms and food factories. Health experts point to the role it plays in human digestion—especially as a byproduct of certain gut bacteria breaking down fiber. Research published in Nature Reviews Gastroenterology & Hepatology shows it strengthens the gut lining, helps manage inflammation, and could possibly lower the risk of colon cancer. This has triggered a wave of butyrate-rich supplements, promising smoother digestion and a stronger immune system. I’ve felt the difference myself after dialing up the fiber in my meals. Bloating and discomfort ease up, proving that sometimes the best results come from what goes unseen, or even unnoticed.
Environmental Shortcuts and Pain Points
Purifying or producing butyric acid at industrial scale isn’t always pretty. Some plants generate odors strong enough to clear out a city block. Proper filtration and closed systems go a long way, but slip-ups still happen. Sustainable plant-based alternatives can help, especially as the demand for animal feed and flavorings rises worldwide. The push toward cleaner, renewable sources comes from both regulators and conscious buyers. Every step cuts environmental headaches for nearby communities and factory workers alike.
Toward Safer, Smarter Use
Butyric acid hides behind powerful associations—good health, fast growth, tasty food—but it also brings challenges. Strong smells, contamination risks, and overexposure in food manufacturing have driven the search for better handling rules. Regular inspection and employee training keep work sites safer. Simple precautions—gloves, masks—limit contact for people mixing and moving it. Technology opens options for greener production using bacteria, slashing waste and pollution at the same time.
The Bigger Picture
This compound delivers bang for the buck in many sectors. Instead of just viewing butyric acid as a bad smell with a fancy name, more eyes now see its part in the cycle from plant to animal to food and back again. Stronger rules, cleaner factories, and an eye for gut health—these all point to a future where the benefits outweigh the stink.
Is butyric acid safe for human consumption?
A Closer Look at a Funky Smelling Compound
Anyone who’s ever opened old cheddar or caught a whiff from a gym sock knows butyric acid, even without the name. The pungent smell comes straight from this short-chain fatty acid, which pops up naturally in butter, some cheeses, and fermented foods. So if something so smelly appears in everyday eats, can it be safe to put in our bodies?
Found Everywhere: From Food to Your Gut
Butyric acid isn’t some novelty cooked up in a lab. Butter gets its name from butyric acid, the compound that offers that sharp tang when you bite into blue cheese or Parmesan. Gut bacteria break down fiber from veggies and whole grains, turning them into this same acid. I’ve seen research published in the American Journal of Clinical Nutrition showing that butyric acid plays a hand in gut health, helping to keep inflammation down and even feeding the cells lining our colon.
Consistency shows up across food science. The U.S. Food and Drug Administration (FDA) lists butyric acid as a substance generally recognized as safe (GRAS) for its use as a flavor agent. And no, the whiff doesn’t linger after eating: our bodies usually process and absorb it well, with most traces gone within hours.
Safety: A Matter of Quantity and Source
No question, the natural sources and small amounts in cheese fill an important nutritional role. But concentrated supplements, which have cropped up in recent years making all sorts of promises about weight loss and gut support, need a closer look. The difference between butyric acid in your sauerkraut and chugging a purified capsule lies in both dose and how your body takes it in.
Anyone thinking about supplements should glance at the science. Some studies find positive effects for people with certain digestive issues, like irritable bowel syndrome, but these benefits show up with medical supervision—not from randomly picking up a supplement. Overdoing concentrated butyric acid, especially without guidance, can mean nausea or gut trouble. Reports in toxicology journals note large doses may irritate the stomach lining, trigger headaches, or mess with the sense of taste.
The Need for Reliable Information
Not all online sources give a fair picture. I’ve seen wellness sites push butyric acid pills with little evidence, while skipping over possible risks. Strong, trustworthy science comes from controlled studies and medical experts, not influencer videos or anecdotal stories. Authority matters here. Dietitians and gastroenterologists who spend years studying the gut know best.
Product purity also counts. Food-grade butyric acid, handled by trained manufacturers, follows strict standards for contents and microbes. Industrial-grade butyric acid isn’t for human consumption. If you buy supplements or additive ingredients off the internet, you might roll the dice with quality.
Choosing Smarter Ways to Support Health
The sensible route taps into food science and brings gut-friendly butyric acid into the diet the old-fashioned way: eating more fiber, fermented foods, butter, and hard cheeses. Enjoying kimchi or sourdough doesn’t just taste good—your colon gets a boost. Anyone thinking about high-dose butyric acid beyond normal food should run plans by a healthcare provider. Everyone’s biology handles things differently, so what’s safe for one isn’t safe for all.
Butyric acid proves that something notorious for its smell easily belongs in a healthy diet, as long as we keep the facts clear and watch the source and dose. As with any health trend, the best path starts with real food and expert advice.
What are the health benefits of butyric acid?
Digestion Gets a Helping Hand
Think about those times when a meal just settles right. No bloating, no discomfort—just easy digestion. Butyric acid, a short-chain fatty acid produced in the colon, plays a big part here. Bacteria in the gut break down fiber from foods like oats, bananas, or onions, creating this compound as a byproduct. Butyric acid becomes the main fuel for cells lining your colon. With it around, the gut lining stays healthy and strong, guarding against unwanted invaders and reducing irritation.
Lowering Inflammation in the Gut
Irritable bowel or chronic gut discomfort can knock anyone out of rhythm. Researchers at the Cleveland Clinic note that butyric acid helps tamp down inflammation, especially in the colon. Crohn’s disease and ulcerative colitis both involve a restless, inflamed gut lining. Animal studies and a handful of human trials have noticed that butyrate enema treatments may help calm gut fires in these conditions by soothing immune cells and encouraging repair work on damaged tissue.
Fueling a Healthy Microbiome
A balanced gut isn’t a guarantee—diet, stress, or antibiotics can tip things off-kilter. Butyric acid can tip it back. A diet high in resistant starch, like what’s found in cooked and cooled rice or potatoes, sends more fuel to good bacteria. More good bacteria means more butyrate, and that means a tougher barrier against nasty bugs. Harvard’s T.H. Chan School of Public Health connects high butyrate levels with fewer infectious invaders breaching the colon lining.
Benefits Stretch Beyond the Gut
The advantages of butyric acid don’t stop with digestion. New studies out of Johns Hopkins show promising results for mental health. The gut and brain share a two-way radio—when the gut feels better, the mind often follows. Butyric acid influences the production of neurotransmitters, which play a role in mood. Some early research even links higher butyrate levels with less anxiety and clearer thinking.
Blood sugar control also ties in. Butyric acid seems to help with insulin response. People eating foods rich in dietary fiber have a lower risk for type 2 diabetes, and scientists believe that the butyrate boost is a big reason for that improved sensitivity.
Getting More Butyric Acid Naturally
The body can make butyric acid, but the building blocks have to show up on your plate. Fiber-rich foods top the list: beans, whole grains, and certain vegetables. Fermented foods like sauerkraut and hard cheeses deliver a direct hit of butyric acid too. I’ve noticed after periods of focused eating—more lentils, more leafy greens—digestive comfort becomes the new normal. Go slow, though; suddenly loading up on fiber can mean some gurgling at first.
Doctors and dietitians agree on the basics: more real, unprocessed plants, less ultra-refined fare. Consistency pays off for gut health, butyric acid production, and overall well-being. If uncomfortable digestive symptoms persist or there’s a diagnosis like IBD, working with a healthcare team lets you find the right approach—sometimes bringing supplements into the mix, sometimes tweaking the plate.
Butyric acid isn’t a magic bullet, but its benefits ripple through many corners of health. Focusing on what ends up in your bowl goes a long way in building a stronger, more resilient gut and, by extension, a happier body.
Where can I buy butyric acid?
Understanding Butyric Acid's Role and Risks
Butyric acid has a place in both science labs and some specialized industries. Anyone who’s come across the intense, memorable odor of butter gone wrong knows just how strong this chemical can be. While some researchers use it to study gut health or fermentation, hobbyists might think about it for things as broad as entomology or home chemistry experiments. Butyric acid isn’t something you’ll see on grocery shelves, and there are some important reasons for that.
Where Real People Actually Find Butyric Acid
You start searching. Popular online marketplaces like Amazon and eBay don’t list pure butyric acid for consumers, probably for good reason—shipping regulations and potential misuse mean most sellers either won’t offer it at all or only supply it to verified professionals. I checked chemical supplier sites such as Sigma-Aldrich, Carolina, and Fisher Scientific, and noticed their ordering process involves paperwork, proof of affiliation with a lab or company, and a shipping address that ties back to an institution. This policy aims to avoid dangerous situations—one spill indoors tells you why.
For smaller quantities, agricultural suppliers use derivatives for animal feed additives, but they rarely sell the concentrated stuff to the public. Garden supply stores occasionally stock products containing butyric acid as animal repellents, but pure solutions stay out of reach. That reflects a long, hard-learned lesson about public safety: accidents with concentrated butyric acid end up as both a fire hazard and a bad day for everyone nearby.
Regulations and Common Sense
Governments classify butyric acid as a hazardous material. The US Environmental Protection Agency sets rules about transportation, storage, and disposal. The European Chemicals Agency maintains similar restrictions. Scientists and industry buyers follow strict handling protocols for a reason. The pungent, corrosive liquid burns skin, corrodes surfaces, and releases fumes that cling to clothes. Regulatory barriers aren’t just boxes to check—they’re signals. An average person finding butyric acid outside a proper context should double check the implications.
Supporting Legitimate Needs, Not Reckless Curiosity
Some folks genuinely need butyric acid for agriculture, university research, or medical studies. Those with a documented reason can get the right type and concentration through established chemical suppliers after showing credentials. These suppliers also provide clear instructions on handling, spill response, and disposal so risk stays manageable. Without formal training or an urgent use, curiosity shouldn’t prompt a purchase.
History teaches hard lessons about chemicals sold without limits. Testimonials from fire departments or restoration crews after accidental spills highlight why open sales don’t replace responsible stewardship. A splash on skin leaves burns, and rooms contaminated with fumes sometimes need days of cleaning.
Safer Alternatives and Going Through the Proper Channels
Anyone trying to replicate laboratory results or use butyric acid in a home experiment might consider reaching out to a university or community lab first. Many student or hobbyist projects succeed using milder acids or food-safe substitutes. Teachers, scientists, and local experts often suggest safer approaches that teach the same lesson while keeping people out of trouble. If a genuine need for the chemical exists, buying through the correct channels, using personal protective gear, and having emergency procedures ironed out remains fundamental.
Access to potent chemicals carries responsibilities. Regulations exist to protect health, air quality, and local communities. Chasing a fast online purchase may look easy, but there’s seldom a shortcut worth the risk—trust the process, and trust the people who’ve worked safely with these substances before.
What are the side effects of butyric acid?
What Butyric Acid Really Is
Butyric acid shows up in some pretty unexpected places. It’s in butter, cheese, and human gut bacteria crank it out when they break down dietary fiber. Food developers use it as a flavor enhancer—though the smell can seem a lot like vomit if you sniff a concentrated batch. In labs and supplements, butyric acid grabs attention as a possible gut health booster. It has a reputation for helping to keep the lining of the colon healthy and supporting immune function.
Side Effects Nobody Warned Me About
I once made the mistake of handling pure butyric acid in a university chemistry class, thinking nothing of it. I never forgot that stinging, foul odor. Touching the undiluted stuff, even just a drop, left a burning, itching sensation on my skin. After hours, the irritation lingered. That’s what concentrated butyric acid can do—burns, redness, and in rare cases, blistering. Eye exposure? You’re signing up for watering, burning, and sometimes corneal injury if you don’t rinse it out immediately.
It’s tempting to ignore the warnings on supplement labels, especially when social media boasts about “miracle” results for digestion or metabolism. Still, I’ve heard people complain of nausea and stomach pain after taking too many butyric acid capsules. Some even get diarrhea, bloating, or a gassy, uncomfortable feeling. Heartburn hits certain folks right after their dose. No one really talks about headaches or the sense of fatigue that can roll in with digestive upset, but it does happen, and it’s worth mentioning for those who feel blindsided.
Inhaling Butyric Acid: Not a Good Idea
Butyric acid vapor sneaks into the nose if you open a container indoors. Even a small whiff causes throat irritation, watery eyes, and heavy coughing—it’s like breathing in tear gas from a kitchen setting. High doses of vapor trigger dizziness and breathing trouble. Not many people mess with pure butyric acid outside a lab, but industrial settings have documented these kinds of reactions, and they’re not pretty.
What the Experts and Research Say
I look to clinical data before buying into supplement hype. The European Food Safety Authority (EFSA) reviewed butyric acid’s safety in animal feed and noted high doses can irritate the digestive system, while the U.S. National Institutes of Health classified it as “generally recognized as safe” if consumed in food amounts. Problems pop up with larger doses, especially above what you’d normally get from food. In allergy-prone people, even low exposures can spark skin rashes or asthma-like reactions.
How to Minimize Risk
Most folks do well with small, food-level doses of butyric acid. If you’re curious about supplements, start with the lowest dose and listen to your body. Skip them if you’re pregnant, breastfeeding, or dealing with active stomach ulcers, unless a doctor says otherwise. Protective gloves and proper ventilation matter for anyone using liquid butyric acid in a lab or bakery. Store it out of reach of pets and children. Proper handling and dosage go a long way to keeping this chemical useful—and not a source of regret.
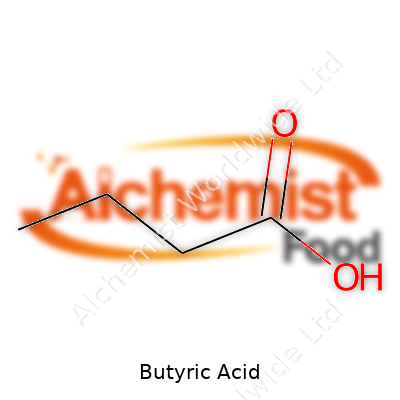
| Names | |
| Preferred IUPAC name | Butanoic acid |
| Other names |
Butanoic acid n-Butyric acid Butanoate Butyrate |
| Pronunciation | /ˈbjuː.tɪr.ɪk ˈæs.ɪd/ |
| Preferred IUPAC name | butanoic acid |
| Other names |
Butanoic acid n-Butanoic acid Buttersäure Buttersaeure Propanecarboxylic acid |
| Pronunciation | /ˈbjuː.tɪr.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 107-92-6 |
| Beilstein Reference | 635 |
| ChEBI | CHEBI:30772 |
| ChEMBL | CHEMBL418 |
| ChemSpider | 259 |
| DrugBank | DB04210 |
| ECHA InfoCard | 100.003.285 |
| EC Number | EC 200-532-5 |
| Gmelin Reference | 543 |
| KEGG | C00246 |
| MeSH | D002073 |
| PubChem CID | 264 |
| RTECS number | EO1400000 |
| UNII | 3G6A5W338E |
| UN number | UN2820 |
| CAS Number | 107-92-6 |
| Beilstein Reference | 1209242 |
| ChEBI | CHEBI:30772 |
| ChEMBL | CHEMBL418 |
| ChemSpider | 259 |
| DrugBank | DB04204 |
| ECHA InfoCard | 100.003.304 |
| EC Number | EC 200-532-5 |
| Gmelin Reference | Gmelin Reference: **1241** |
| KEGG | C00246 |
| MeSH | D001570 |
| PubChem CID | 264 |
| RTECS number | EB5425000 |
| UNII | 3G6A5W338E |
| UN number | UN2820 |
| Properties | |
| Chemical formula | C4H8O2 |
| Molar mass | 88.11 g/mol |
| Appearance | Colorless to light yellow liquid with an unpleasant, rancid odor |
| Odor | Unpleasant, rancid, butter-like |
| Density | 0.96 g/cm3 |
| Solubility in water | Miscible |
| log P | 0.79 |
| Vapor pressure | 0.43 mmHg (20 °C) |
| Acidity (pKa) | 4.82 |
| Basicity (pKb) | pKb ≈ 10.24 |
| Magnetic susceptibility (χ) | -6.28×10⁻⁶ |
| Refractive index (nD) | 1.399 |
| Viscosity | 2.2 mPa·s (25 °C) |
| Dipole moment | 1.70 D |
| Chemical formula | C4H8O2 |
| Molar mass | 88.11 g/mol |
| Appearance | Colorless to light yellow oily liquid with an unpleasant, rancid odor |
| Odor | Rancid, unpleasant, butter-like |
| Density | 0.96 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.79 |
| Vapor pressure | 0.43 mmHg (20°C) |
| Acidity (pKa) | 4.82 |
| Basicity (pKb) | 4.83 |
| Magnetic susceptibility (χ) | -6.41×10⁻⁶ |
| Refractive index (nD) | 1.399 |
| Viscosity | 2.59 mPa·s (at 25 °C) |
| Dipole moment | 1.91 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 152.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -482.60 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –2186 kJ·mol⁻¹ |
| Std molar entropy (S⦵298) | 158.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -482.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2276 kJ/mol |
| Pharmacology | |
| ATC code | A16AX01 |
| ATC code | A07CC05 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02, GHS05, GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314, H332 |
| Precautionary statements | P210, P280, P301+P310, P305+P351+P338, P331 |
| NFPA 704 (fire diamond) | 2-3-2-W |
| Flash point | 72 °C |
| Autoignition temperature | 343 °C |
| Explosive limits | 2–10% |
| Lethal dose or concentration | LD50 oral rat 2,940 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 2,940 mg/kg |
| NIOSH | BUA |
| PEL (Permissible) | 50 ppm |
| REL (Recommended) | 15 mg |
| IDLH (Immediate danger) | 200 ppm |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02, GHS05, GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H302, H314, H332 |
| Precautionary statements | P210, P260, P264, P280, P301+P330+P331, P303+P361+P353, P305+P351+P338, P310, P321, P363, P370+P378, P403+P233, P501 |
| NFPA 704 (fire diamond) | 3-2-0-SA |
| Flash point | 72 °C |
| Autoignition temperature | 310 °C |
| Explosive limits | 1.8–10.9% |
| Lethal dose or concentration | LD50 oral rat 2,940 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Butyric Acid: "2,940 mg/kg (rat, oral) |
| NIOSH | BUA |
| PEL (Permissible) | 50 ppm |
| REL (Recommended) | 500 mg |
| IDLH (Immediate danger) | 200 ppm |
| Related compounds | |
| Related compounds |
Acetic acid Propionic acid Valeric acid Isobutyric acid Caproic acid |
| Related compounds |
Butyric anhydride Butyryl chloride Ethyl butyrate Methyl butyrate |

