Ammonium Hydrogen Carbonate: A Closer Look at Its Role and Outlook
Historical Development
Ammonium hydrogen carbonate traces its origins back several centuries, long before modern chemistry gave things tidy labels. Early bakers and apothecaries in Europe called it "hartshorn" because they heated animal horns to collect the pungent-smelling powder. This "volatile salt of ammonia" helped European cookies and cakes puff up years before baking powder hit store shelves. As the chemical industry advanced during the 19th century, companies started producing it by more predictable synthetic routes, giving bakeries and laboratories a steady supply. Over time, food-safe regulations replaced folk knowledge, but the compound kept earning a spot in both pantry and lab for its reliability and simplicity.
Product Overview
Ammonium hydrogen carbonate shows up as a white, crystalline powder with a sharp, ammoniacal odor that’s unmistakable once you’ve worked with it. Commonly found under names like ammonium bicarbonate or baking ammonia, it carries the chemical formula NH4HCO3. Some people call it "powdered baking ammonia" in kitchens and "ABC" shorthand in technical shops. Its use in baking, cleaning, and chemical processes makes it a familiar item in a surprising number of industries. Whether you’re whipping up crisp cookies or running analysis in a quality-control lab, chances are you’ve brushed up against it.
Physical & Chemical Properties
This salt dissolves readily in water, throwing off that classic “cleaner” smell as ammonia gas escapes, especially when warmed. At room temperature, the powder looks harmless, but heating to about 36°C (around body temperature) begins breaking it down into ammonia, carbon dioxide, and water vapor. Storage needs to be in a cool, dry spot, since moisture and warmth spark decomposition and result in clumping or loss of leavening power. Chemically, ammonium hydrogen carbonate plays both sides: it acts like a mild base, and its tendency to decompose easily makes it perfect for applications needing fast-release leavening or controlled pH increases. Most grades land between 99% and 100% purity; food and technical requirements set thresholds for contaminants like heavy metals and chlorides.
Technical Specifications & Labeling
Pure ammonium hydrogen carbonate marketed for baking or food processing is listed as E503(ii), and labeled clearly for ingredients and safe storage. Specifications require a dense, white powder, usually with impurities measured in parts per million. Lot numbers, manufacturer details, and recommended storage temperatures (between 15°C and 25°C, in hermetically sealed vessels) commonly appear. Packaging for commercial bakeries often runs between 25 kg and 50 kg sacks, while laboratory suppliers usually bottle it in small quantities with tight lids to check gas loss and moisture. Food-grade lots exclude detectable levels of toxic elements including arsenic or lead, aligning with local food safety laws. Chemists and handlers count on valid certificates of analysis, as trace contaminants can ruin final products or create batch inconsistencies.
Preparation Method
Factories typically make ammonium hydrogen carbonate by passing carbon dioxide through an aqueous solution of ammonia. CO2 dissolves in the solution; ammonia binds with it and water to form NH4HCO3. Cooling the solution crystallizes the salt out. The process allows industrial-scale production as long as there’s careful temperature control because excess warmth breaks down the product before it even leaves the factory. Some old-school handbooks mention using the distillation of horn or hoof shavings, but commercial supply relies on synthetic routes for consistency and purity. Modern operations keep the same principle—ammonia gas, water, and carbon dioxide, just in controlled tanks with sensors, rather than backyard stills.
Chemical Reactions & Modifications
In my own lab experience, heating ammonium hydrogen carbonate is like releasing bottled fizz—the salt decomposes, shooting out ammonia and carbon dioxide rapidly, often leaving no residue behind when used in baking. Acidic ingredients in recipes or industrial mixtures drive even faster breakdown. This property makes the compound shine as both leavening and pH adjuster. On the chemical stage, it opens the door for more complex ammonium salts; for example, mixing with further acids yields different ammonium-based salts with unique behaviors. Dry heating in the absence of acid simply gives off gases and leaves nothing but air. So far, few practical modifications have replaced the original salt’s quick release and clean end-products, meaning it keeps hold of niche uses.
Synonyms & Product Names
Walk into a bakery supply shop or chemical distributor, and this compound will answer to several names. In the baking aisles, people use "baking ammonia," "bakers’ ammonia," or "hartshorn." Scientific catalogs print the systematic name or simply "ammonium bicarbonate", even though technically the chemical structure better fits the "hydrogen carbonate" name. On shipping manifests and technical labels, you’ll spot codes or abbreviations like ABC. Commonality between food and reagent grades keeps confusion minimal. Those in food production and research tend to favor specific labeling to avoid regulatory headaches, while seasoned technicians just call it what their first boss taught them.
Safety & Operational Standards
Working with ammonium hydrogen carbonate doesn’t spark panic, but sensible measures keep staff and products safe. Direct skin contact may irritate sensitive users, and inhaling the powder, or its released ammonia fumes, gets unpleasant fast—those headaches and watery eyes are nobody’s idea of fun. OSHA and EU guidelines recommend gloves, fume hoods, and dust masks for large-scale handling. Proper ventilation keeps air clean in busy bakeries that use the compound all day, and food plants regularly inspect storage rooms to avoid cross-contamination. Even at home, spirits should never add it to food that isn’t baked off, since undecomposed powder and released ammonia carry risks for stomach and lungs. Disposal follows local hazardous chemical rules, with small quantities permitted for landfill but larger amounts managed by chemical waste services.
Application Area
Food production represents the busiest sector for ammonium hydrogen carbonate. Bakeries pick it for traditional recipes where a crisp, airy texture counts—think European cookies, crackers, and spiced rusks. Since the compound decomposes fully in dry heat, no chemical taste lingers in the pastry. Chefs sometimes prefer it for those flat, snappy baked goods you find at Christmas markets. Its applications don’t stop at food: cleaners and detergents use it for controlled pH adjustment and gentle scouring, while analytical chemists depend on its volatility to calibrate some lab tests. In agriculture, companies sometimes turn to it for fertilizer blends, as it delivers a rapid nitrogen boost without stubborn chemical residues. The compound shows up in fire extinguisher powders and as a component in dyeing and tanning. Despite calls for alternatives, ammonium hydrogen carbonate still fills its unique corner.
Research & Development
Recent research explores ways to improve the compound’s stability and expand its food-safe alternatives. Food technologists test blends combining ammonium hydrogen carbonate with modern leavening acids to fit new processing lines. Analytical scientists study its thermal decomposition to fine-tune mass spectrometer calibrations and reference patterns. Some chemical manufacturers have invested in developing encapsulated versions, hoping to delay its gas release until high heat hits—a trick that could help in high-moisture baked goods. Environmental researchers also examine its breakdown products to confirm no harmful residues end up in food, soil, or water, which keeps regulatory bodies informed and market approvals in place.
Toxicity Research
Toxicological testing shows ammonium hydrogen carbonate to be relatively low-risk in expected food and industrial doses. Once baked, fumes and volatilized salt mostly escape, cutting exposure. High concentrations or chronic inhalation—think uncovered industrial spills or factory mishaps—cause nose, throat, and lung irritation in workers. Ingesting large uncooked amounts can lead to nausea or damage, especially for children, pets, or curious handlers not reading labels. Animal studies and food safety reviews confirm safe use at regulated levels, with strict limits set for unreacted residues in finished foods. Most countries’ food safety agencies stick by the same verdict: used correctly, with ventilation and clear labeling, the compound poses no threat to public or worker health. Ongoing occupational health monitoring confirms these habits keep risk low, but regular updates to standards reflect new findings.
Future Prospects
Looking ahead, ammonium hydrogen carbonate will likely keep its slot in traditional baking and laboratory settings, even as new leavening agents and fertilizer formulas emerge. Process improvements target better storage stability, smarter dosing, and easier handling to manage product loss and odor. In food, consumer trends toward nostalgia and heritage recipes keep demand steady, especially where no one wants modern substitutes carving out flavor or texture. Environmental advocates push for tighter emission standards and greener sourcing of ammonia and carbon dioxide inputs, prompting chemical firms to tweak process design. Biotechnology sectors eye the compound for novel uses, such as nitrogen source in certain fermentation pathways. Continuous testing and regulatory review ensure peace of mind for health and environmental impact. With each batch, new hands in the industry learn it works reliably where nothing else quite does, so its story, old as it is, isn’t fading from the scene soon.
What is Ammonium Hydrogen Carbonate used for?
Baking and the Magic of Puff
Walk into a bakery and sniff the air. There’s a good chance ammonium hydrogen carbonate played a role in making all those pastries and cookies rise so high. Bakers call it “baker’s ammonia,” and it’s been around for centuries. I remember my grandmother sprinkling it into her holiday cookie dough, a trick she learned overseas. In old recipes for flat cookies like Springerle or certain crackers, it gives a crisp finish and a light bite. Heat triggers it to release ammonia and carbon dioxide—leaving no trace behind except for the tender texture of baked treats.
Industrial Food Production
The baking shelf isn’t the only place you’ll find ammonium hydrogen carbonate. Food factories use it in large-scale cookie or biscuit production. Its main appeal comes from how clean it works: it leaves zero salty aftertaste or leftover chemicals. I once visited a biscuit plant in Poland where this ingredient flowed by the bagful into mixing machines. The manager told me their decades-old recipes still rely on it, especially for thin items where baking soda just won’t do the job right. European Union guidelines approve its use (labeled as E503), and food scientists keep choosing it for the consistent, zippy rise it brings.
Beyond Baking: Agriculture and Cleaning
It’s not all cake and cookies. In greenhouses, ammonium hydrogen carbonate feeds plants by gently raising nitrogen levels without burning the roots. Vegetable growers sometimes sprinkle it to replenish tired soil. Farmers know their trade, and if a trick works, they keep it in the toolbox. I saw this firsthand during an internship at a horticulture research facility; the head grower swore it kept the lettuce crisp and healthy when synthetic fertilizers became too pricy.
You’ll find it in cleaning, too. Ammonium hydrogen carbonate makes a mild scouring powder that handles stains and mineral deposits without scratching sensitive surfaces. Some specialty labs use it because it leaves no hard-to-remove residue. There’s satisfaction in cleaning with an agent that disappears entirely, especially in places where traces might taint food or pharmaceutical products.
Health, Safety, and Regulations
People sometimes worry about what goes into their food. Ammonium hydrogen carbonate’s safety profile is solid when used as intended, but proper ventilation makes sense, especially in places where ammonia could build up—they don’t bake in windowless closets for a reason. The ingredient breaks down completely at normal baking temperatures, so no one ends up eating or inhaling leftover ammonia. Regulatory agencies in the US, Europe, and much of the world have green-lit its usual food applications. Still, as with any food additive, it’s smart to pay attention to quality and source, especially for anyone dealing with health conditions or allergies.
Looking to the Future
As people search for food ingredients with a history and a light environmental footprint, traditional helpers like ammonium hydrogen carbonate often stand out. It comes from simple raw materials: ammonia, carbon dioxide, and water. Modern research keeps tabs on emissions to make sure production stays clean. For bakers, chemists, and farmers, knowing the science behind what they use helps build trust and better results.
Is Ammonium Hydrogen Carbonate safe for food applications?
Understanding the Role in Baking
Anyone who enjoys baking biscuits or crunchy cookies has likely benefitted from a chemical reaction involving ammonium hydrogen carbonate. Baker’s ammonia, as it’s often called, has been on ingredient lists for generations. Used mainly in low-moisture baked goods, it helps dough rise quickly and creates that signature crisp texture. After heating, it breaks down into three simple components: ammonia gas, carbon dioxide, and water vapor. This isn’t just food science trivia — these gases escape during baking, leaving behind no trace of the original compound.
Safety Backed by Science and Regulation
Food safety matters, especially with less familiar ingredients. Health authorities like the U.S. Food and Drug Administration and the European Food Safety Authority both approve ammonium hydrogen carbonate for use in food. They’ve reviewed thousands of cases and haven’t seen evidence that occasional ingestion in typical amounts poses a hazard.
Ammonium compounds break apart and leave no toxic residue, provided foods are baked thoroughly. That’s an important distinction. If not cooked enough, ammonia can linger and leave an unpleasant odor, though there’s little evidence this would pose much risk. For sensitive groups, such as those with kidney disease, pharmacists sometimes advise caution with any ammonium salt, but for most people, it doesn’t build up or cause trouble.
Why Scrutiny Matters
Transparency builds trust. People care deeply about what goes into their food—rightly so. Reports about chemical-sounding names often spark fear, sometimes justified, sometimes not. But unlike additives that stick around or accumulate, ammonium hydrogen carbonate doesn’t settle in the body. Data from the Joint FAO/WHO Expert Committee on Food Additives lists it among the safest leaveners.
Still, anyone with asthma or a very sensitive respiratory tract could notice a harsh smell if using it at home in large quantities or in cramped kitchens. Most industrial bakeries manage this risk by using good ventilation systems and precise dosing.
Common Questions and Practical Answers
A recurring worry involves possible contamination or the formation of harmful byproducts. Food-grade ammonium hydrogen carbonate passes through strict manufacturing checks for purity. Research has shown that at the temperatures found in commercial baking, no harmful residues end up in finished products. Homemade recipes should stick to recommended amounts and proper baking temperatures — which takes care of ammonia odor and ensures safety.
Some folks with a history of allergies wonder about hidden risks. The compound itself isn’t an allergen, but checking for cross-contamination in bulk ingredients never hurts. Gluten-free recipes use ammonium hydrogen carbonate to achieve crunchiness that’s hard to match otherwise.
Balancing Innovation and Tradition
Modern bakers have choices. Sodium bicarbonate (baking soda) and baking powder work for most recipes, but none replace the crisp, open texture from baker’s ammonia in certain cookies. That said, for cakes or breads, ammonium hydrogen carbonate doesn’t fit — it can leave a harsh flavor if moisture doesn’t evaporate.
Staying informed means reading labels, following reliable recipes, and not fearing a name just because it sounds chemical. Ammonium hydrogen carbonate remains a safe, well-researched part of traditional and modern baking worldwide. Food safety relies on informed choices, good process, and ongoing scientific review. For those who love old-school crispness in baked treats, this trusted ingredient isn’t going anywhere soon.
How should Ammonium Hydrogen Carbonate be stored?
Why Handling Makes the Difference
Ammonium hydrogen carbonate doesn’t stir up much attention until someone walks into a supply closet and finds a strange ammonia smell hanging in the air. I recall a bakery in my early years. The head baker kept the carton on a high shelf near a window, right above a radiator. After a week, most of the powder had vanished, leaving caked residue and that unmistakable scent. People laugh until they remember their own first time. Experience teaches you that chemicals like this react to their surroundings. Ammonium hydrogen carbonate breaks down with warmth and moisture, releasing ammonia gas. So, nothing beats a dry, cool, and airtight spot for storage.
Common Mistakes and Hidden Costs
Shoving the container into a random cupboard, maybe next to cleaning supplies or acids, causes headaches. If moisture slips in, the powder lumps or loses strength. Tossing it next to acids? That just produces irritating fumes. Plenty of food factories learn lessons the hard way, spending afternoons airing out rooms or tossing out wasted product. A single slip can mean lost inventory and extra cleaning, especially in high-humidity regions during summer.
Learning from Everyday Spaces
Look around most kitchens, bakeries, or labs, and you’ll spot chemical storage blunders. Shelves near dishwashers, fridges leaking condensation, employees sneaking in hot coffee — all of these undermine safe storage. My neighbor once stashed ammonium hydrogen carbonate over his oven, only to find strange crystals forming in the jar after a month. Even dry storerooms need low humidity and steady temperature to prevent unpredictable changes in the chemical. Using sealed, labeled containers isn’t about appearances; it shields the powder from invisible dangers. Quality airtight jars or bags keep out moisture, air, and obviously children or pets.
Safety Isn't Overkill
It pays to talk about personal safety. The dust irritates the eyes and nose, especially in drafty environments or during repackaging. Splashing a little on skin causes itchiness. So, gloves and safety glasses aren’t tools for the timid; they stop unwanted medical visits. Any room where ammonium hydrogen carbonate gets stored should have basic ventilation, though no one wants to create wind tunnels. Regularly checking the storage date goes a long way too — once it’s caked, it’s done.
Rules Worth Following
Regulators require food-grade ammonium hydrogen carbonate to live in labeled, closed packaging. This rule isn’t made by lawyers sitting around a table — it comes from incidents piling up. The European Food Safety Authority, for example, stresses purity and no cross-contamination. In China and across Europe, routine inspections keep suppliers honest. Companies that skimp on proper storage see declines in product quality, face fines, or lose customers’ trust.
Solutions Rooted in Common Sense
Solutions start simple: keep the chemical away from heat and sun, inside storage designed for powdered chemicals. Investing in inexpensive dehumidifiers for humid climates pays off quickly. Staff training shouldn’t feel like a burden—it protects business and health. Clear signage, regular checks for leaks, and using the oldest stock first go further than fancy equipment. These habits mean less waste and fewer problems, both at work and at home. Care with storage frees up time for better things than cleaning up after a chemical mishap.
What are the main differences between Ammonium Hydrogen Carbonate and baking soda?
Unpacking Ingredients: How They Stand Apart
Most kitchen cupboards hold a box of baking soda, but only a few people outside the food industry ever see ammonium hydrogen carbonate. Both seem simple on paper, but real differences start at the chemical level and ripple through to their uses. Baking soda—also known as sodium bicarbonate—shows up in countless homes for cleaning, cooking, and even as a quick fix for heartburn. Ammonium hydrogen carbonate appears mostly in food processing and specialty baking, and its presence at home is rare for a good reason.
Baking soda enters the spotlight in home kitchens due to reliability and safety. It reacts with acids in doughs or batters, releasing carbon dioxide slowly and lifting cakes or cookies into light, airy textures. Ammonium hydrogen carbonate, on the other hand, breaks down when heated and produces carbon dioxide rapidly—but it brings along ammonia gas. That ammonia gives efficiency in leavening, particularly for low-moisture, crisp pastries. But it leaves an unmistakable, unpleasant taste if not baked out completely. No one wants that in their chocolate chip cookies.
Health and Safety: What Gets to the Table
Regulators have tested both ingredients for food safety. Sodium bicarbonate holds the advantage, earning trust for general consumption. Health agencies such as the U.S. Food and Drug Administration classify baking soda as 'generally recognized as safe' for a wide range of uses—not only baking. It can touch everything from medicine to cleaning without much fuss.
Ammonium hydrogen carbonate sits in the shadows. Its ammonia byproduct sends up red flags. Ammonia gas can make people cough and turns stomachs even in small traces. That's why bakeries use it only in certain recipes, and regulations limit its presence in food. I grew up helping in a European bakery where puff pastry used to get a boost from this chemical—always with strict directions to keep the layers thin and temperatures high so the gas escaped fully. No shortcuts allowed.
Environmental and Storage Considerations
Shelf stability draws another line between these two. Baking soda lasts forever if sealed and dry, ready for any use you throw at it. Ammonium hydrogen carbonate, on the other hand, starts to break down over time, especially in humid conditions. Bakers store it in tight, cool spaces and toss it at the first whiff of ammonia.
Everyday Usability and Substitution Woes
Baking soda works as a household multitasker—not just for baking, but for cleaning, deodorizing, and soothing minor skin irritations. Ammonium hydrogen carbonate never sees that kind of action. Swap them by accident, and cookies may end up tasting like cleaning fluid. That’s why clear labeling and separate storage matter at every bakery. Most home cooks learn quickly to trust what they know, and baking soda fits smoothly into family traditions worldwide.
Solutions for Safer, Smarter Choices
Bakers, educators, and food safety experts benefit from sharing clear guidance about these leavening agents. Baking classes could cover not only techniques but the chemistry behind each ingredient—for example, showing exactly how heat, moisture, and acidity drive the differences. Policies should continue to promote labeling standards, so consumers never confuse a food-safe ingredient with a specialized product.
Building on facts and real kitchen experiences, people can make better choices for their health and taste buds. Understanding what goes into dough gives everyone the power to bake safely, enjoy new textures, and avoid unpleasant surprises. At the end of the day, that’s something worth celebrating in every kitchen.
What are the safety precautions when handling Ammonium Hydrogen Carbonate?
Looking Closer at What’s in the Bag
Ammonium hydrogen carbonate shows up as a white crystalline powder, often packed in plastic or paper bags. Bakers and food makers use it for leavening, but industries see it as a pH regulator and gas source. Tossing it around without care can put your lungs and skin at risk because dust will spread fast, and it releases ammonia fumes—nobody wants to breathe that in.
Personal Experience Counts
From several kitchen experiments and a couple of warehouse visits, one lesson stands out: ventilation matters. Even small amounts find a way to irritate the throat and eyes. Gloves take priority, especially for anyone spending more than an hour around open containers. Safety glasses stay on, no excuses. That’s not an overreaction—it’s called going home without red, watery eyes and scratchy hands.
Protecting Skin and Eyes
Short sleeves don’t cut it here. Long-sleeved lab coats or coveralls reduce the chance of direct contact. Any exposed bit of skin becomes a target for that alkaline powder. Goggles shield the eyes better than typical glasses, trapping airborne particles and blocking out bursts of powder—you don’t get a second chance if it stings.
Don’t Ignore Respiratory Safety
Grinding, pouring, or mixing ammonium hydrogen carbonate always puts a little in the air. NIOSH-approved dust masks or respirators give lungs a fighting chance. It’s tempting to skip the mask for a quick chore, but just one whiff of ammonia reminds you why the extra layer matters. Sensitivity varies, but nobody enjoys the burning tickle from stray dust or fumes.
Spill Management and Clean-Up Habits
Spills sound harmless, but sweeping kicks dust everywhere. Wet cleaning works better—damp cloths catch more without raising dust clouds. Keeping a spill kit nearby isn’t overkill; it means you grab what’s needed fast. Used cloths, gloves, and masks don’t belong in open bins; seal them in plastic bags to keep residue from escaping. Disposal follows local hazardous waste rules instead of plain trash pick-up.
Storage Isn’t Just Shelf Space
One bad habit involves stacking bags in warm storage rooms. Moisture and heat make ammonium hydrogen carbonate break down, releasing ammonia. That leads to bent shelving, odors, and dangerous buildup. Cool, dry, well-labeled storage spaces slow down that process and let you keep things organized. Dry hands and tools for scooping make a difference too—nobody needs clumps or caked powder blocking scoops and scales.
Preparing for Accidents, Not Just Hoping for Luck
First aid kits in chemical workspaces stock eye wash and mild soap. If someone gets dust in their eye, speed matters; rinse for 15 minutes under running water. Accidental skin contact gets the same soap-and-water treatment. Anyone feeling dizzy or sick from fumes heads outdoors and takes a break in fresh air. Reporting every exposure, even mild ones, helps spot problems before they get worse in the team.
Clear Labels and Shared Information
Routine and trust come from clear hazard labels and regular reminders. Everyone in the workspace needs to know what’s in a container and the fastest exit routes. Posting safety sheets—right where people measure or use the compound—makes it harder to overlook the risks. Training new folks with hands-on demos, not just handouts, ensures those safety rules actually stick.
Picking Smarter Solutions
For anyone seeking alternatives, mechanical aeration and modern leavening systems offer a path away from ammonium hydrogen carbonate in some settings. No one product fits every job, but technology keeps expanding choices for those who care about health, efficiency, and safety. Experience shows that small changes, like sealed dispensing systems, cut back on accidents and protect people who keep our kitchens, bakeries, and labs humming every day.
| Names | |
| Preferred IUPAC name | Azanium hydrogen carbonate |
| Other names |
Ammonium bicarbonate Ammonium acid carbonate Bicarbonate of ammonia |
| Pronunciation | /əˈmoʊniəm ˈhaɪdrɪdʒən kɑːrˈbɒneɪt/ |
| Preferred IUPAC name | Ammonium hydrogen carbonate |
| Other names |
Ammonium bicarbonate Ammonium acid carbonate Bicarbonate of ammonia Ammonium hydrogencarbonate Ammonium hydrogen carbonate |
| Pronunciation | /əˈmoʊ.ni.əm ˈhaɪ.drɪdʒən kɑːrˈbɒn.eɪt/ |
| Identifiers | |
| CAS Number | 1066-33-7 |
| Beilstein Reference | 505830 |
| ChEBI | CHEBI:62990 |
| ChEMBL | CHEMBL1082712 |
| ChemSpider | 54659 |
| DrugBank | DB13024 |
| ECHA InfoCard | 03b1663c-db60-4d96-9dde-9bcc6a6c6343 |
| EC Number | 213-911-5 |
| Gmelin Reference | 109900 |
| KEGG | C01342 |
| MeSH | D000648 |
| PubChem CID | 14013 |
| RTECS number | BQ9825000 |
| UNII | NH4B70I9KN |
| UN number | UN3077 |
| CAS Number | 1066-33-7 |
| Beilstein Reference | 3587159 |
| ChEBI | CHEBI:62955 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 54655 |
| DrugBank | DB04345 |
| ECHA InfoCard | ECHA InfoCard: 028-018-00-1 |
| EC Number | 213-911-5 |
| Gmelin Reference | 75494 |
| KEGG | C14416 |
| MeSH | D000647 |
| PubChem CID | 14013 |
| RTECS number | BP6860000 |
| UNII | 49K780UN6T |
| UN number | UN3077 |
| Properties | |
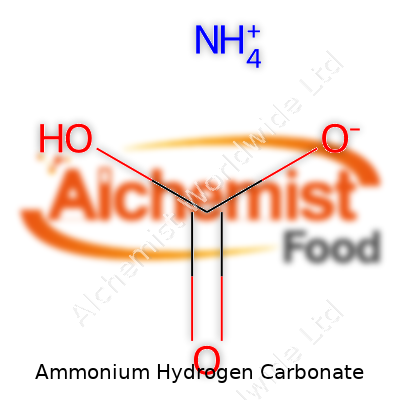
| Chemical formula | NH4HCO3 |
| Molar mass | 79.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Ammonia odor |
| Density | 1.59 g/cm³ |
| Solubility in water | 17.4 g/100 mL (25 °C) |
| log P | -4.61 |
| Vapor pressure | Negligible |
| Acidity (pKa) | NH4+: 9.21, HCO3-: 10.3 |
| Basicity (pKb) | 7.19 |
| Magnetic susceptibility (χ) | -38.0e-6 cgs |
| Refractive index (nD) | 1.409 |
| Dipole moment | 3.5 D |
| Chemical formula | NH4HCO3 |
| Molar mass | 79.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Ammonia odor |
| Density | 1.59 g/cm³ |
| Solubility in water | 17.4 g/100 mL (25 °C) |
| log P | -4.59 |
| Vapor pressure | 17.6 mmHg (20°C) |
| Acidity (pKa) | 15.4 |
| Basicity (pKb) | 7.19 |
| Magnetic susceptibility (χ) | -47.0e-6 cm³/mol |
| Refractive index (nD) | 1.398 |
| Dipole moment | 4.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 216.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -897.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -873.8 kJ/mol |
| Std molar entropy (S⦵298) | 217.1 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -807.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -949.8 kJ/mol |
| Pharmacology | |
| ATC code | A12BA04 |
| ATC code | A12AA08 |
| Hazards | |
| GHS labelling | GHS07; Warning; H302; P264, P270, P301+P312 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Store in a well-ventilated place. Keep container tightly closed. Store locked up. Dispose of contents/container in accordance with local/regional/national/international regulations. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | > 107°C (225°F) |
| Lethal dose or concentration | LD50 oral rat 1,570 mg/kg |
| LD50 (median dose) | 4570 mg/kg (Rat, oral) |
| NIOSH | SC0350000 |
| PEL (Permissible) | PEL: 50 mg/m³ |
| REL (Recommended) | Bakery: 67-26-00/490 g |
| Main hazards | Hazardous if swallowed, causes irritation to eyes, skin, and respiratory tract. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P264 Wash ... thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P301+P312 IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. P330 Rinse mouth. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 oral rat 1576 mg/kg |
| LD50 (median dose) | 1576 mg/kg (Rat, oral) |
| NIOSH | RN822 |
| REL (Recommended) | 30 mg/m³ |
| Related compounds | |
| Related compounds |
Ammonium carbonate Sodium bicarbonate Ammonium carbamate Ammonium chloride |
| Related compounds |
Ammonium carbonate Sodium bicarbonate Ammonium carbamate Ammonium chloride |

