Ammonium Dihydrogen Phosphate: From Chemistry Roots to Future Innovation
Historical Development
The story of ammonium dihydrogen phosphate, known among chemists as ADP or monoammonium phosphate, weaves through the early days of agricultural chemistry. Researchers in the 19th century began searching for effective, water-soluble ways to deliver phosphorus to growing crops. Early phosphates often sat locked in the soil, so scientists looked for compounds that plants could easily take up. ADP, with its strong solubility and adaptability, gained traction in Europe before finding its way into farms and labs across the world. By the 20th century, large-scale manufacturing gathered pace, supporting food security after world wars and helping transform the yield potential of commercial farming.
Product Overview
Ammonium dihydrogen phosphate fills a niche that extends beyond just fertilizer. Present in fire extinguishers, flame-retardant sprays, yeast nutrient blends, and even optical applications, ADP enters daily life in more ways than most expect. It lands in the form of a crystalline solid, each particle capable of dissolving quickly into solutions or blending efficiently with other granular compounds. My own experience with fertilizers in a small garden taught me that ADP stands out for its speed of action — get it into the soil, and most crops show results within days, particularly leafier greens.
Physical & Chemical Properties
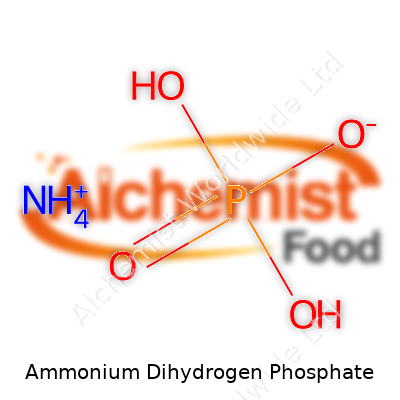
Chemically, ammonium dihydrogen phosphate has the formula NH4H2PO4. Its clear, colorless crystals carry a molecular weight of 115.03 g/mol. Moisture draws towards it, so in humid environments, it clumps unless stored away from air. The melting point, generally around 190°C, sits important for anyone concerned with manufacturing conditions or safe material handling. Dissolve it in water, and you get a mildly acidic solution, something that influences root development in soils as well as various lab-based reactions. Its phosphate content typically runs around 61% (P2O5 equivalent), with nitrogen at roughly 12%, making it a balanced source for both macronutrients.
Technical Specifications & Labeling
Industry standards guide the labeling, with strict requirements for minimum purity, absence of heavy metals, and clear notations on the phosphorus and nitrogen percentage. Bags, whether destined for agricultural or industrial use, arrive stamped with batch numbers, handling directions, safety alerts, recommended storage temperatures, and expiry dates. This traceability becomes vital during audits or accidental spills. Lately, the move toward more transparent supply chains shows customers not just where the bag was filled but often the exact extraction point of the raw phosphate ore — a step towards sustainability and quality assurance.
Preparation Method
The most common route starts from phosphoric acid and ammonia. Mix them in the right proportion: the reaction creates ammonium dihydrogen phosphate as it cools. Industrial plants typically operate in a continuous stream, with excess ammonia sometimes being recycled to reduce waste. From my own experience volunteering at a water-quality lab, I witnessed the importance of quality raw inputs; impurities can carry over, risking contaminant buildup in food chains. Chemical engineers often monitor the crystallization temperature and purity at every stage with spectrometers to guarantee the product meets food safety standards.
Chemical Reactions & Modifications
In a chemical lab, ammonium dihydrogen phosphate sees use in synthesis and as a buffering agent because it reacts predictably under heat or with strong bases and acids. Heat it enough, and ADP loses ammonia, morphing into phosphoric acid. Bring in base, and you might shift the balance toward diammonium phosphate (DAP), which holds a slightly different nutrient ratio and responds differently in soil chemistries. For fire safety applications—think those red sprays in forest firefighting—companies sometimes modify the compound, optimizing stability over range of temperatures or pre-mixing with colorants for better visual tracking.
Synonyms & Product Names
Over the years, ammonium dihydrogen phosphate adopted several identities. Monoammonium phosphate, MAP, NH4H2PO4, ammonium phosphate monobasic—these all refer to the same compound. On international labels or shipping manifests, you’ll see these names, sometimes along with translated terms. Retail fertilizer blends highlight the N-P-K ratio (such as 12-61-0), which helps farmers or gardeners choose the right nutrient mix for their crops. In industrial procurement, clear labeling supports safe handling and lowers the risk of mishaps, as similar-sounding compounds can behave quite differently.
Safety & Operational Standards
Personal experience in a warehouse environment drove home the importance of proper storage for ADP. Although non-flammable and not acutely toxic, the powdery crystals irritate skin, eyes, and respiratory passages during blending or bagging. Good ventilation and protective gear make daily handling far safer. Regulatory agencies—including OSHA and the European Chemicals Agency—track ammonium phosphates; safety data sheets must accompany each batch. These sheets outline first-aid steps, recommended fire-fighting measures, recommended spill cleanup techniques, and advice for disposal, keeping both workers and the environment safeguarded.
Application Area
Farmers value ammonium dihydrogen phosphate for how it supports early root development in crops. Phosphorus takes on outsized importance in seedling stages, and nitrogen gives early shoots a jump start. Outside the fields, ADP’s role in dry chemical fire extinguishers receives less attention, though it’s hard to overstate its life-saving function in stopping kitchen or electrical fires. The food industry uses it as a leavening agent and pH control substance in baking powders. Even in optics, large, single crystals of ADP grow for use as polarizers or frequency doublers in laser technology, highlighting its stretch from the soil to science labs to everyday electronics.
Research & Development
Recent years brought plenty of new attention. Scientists and companies invest in new versions with modified particle sizes for more efficient nutrient delivery, reducing runoff and environmental impact. Some labs experiment with mixed formulations adding trace micronutrients or organic amendments, aiming for stabilization in a wider range of soils. Environmentalists push for slow-release coatings that lower the risk of algal blooms in lakes and rivers, which result from phosphorus leaching. Universities research the effect of ammonium dihydrogen phosphate on soil microbiota, tracking changes in beneficial bacteria or fungi populations that support healthier plants naturally.
Toxicity Research
ADP sits in a relatively benign category, though misuse poses challenges. Inhaling dust causes coughing and throat irritation, and long-term exposure in enclosed spaces might contribute to chronic respiratory issues. The compound breaks down naturally in the environment, but phosphorus runoff into waterways feeds toxic algae, harming fish and drinking water supplies. Researchers now test advanced formulations that curb leaching or tie up phosphorus in plant-available forms for longer. In animal studies, high concentrations may disrupt gut flora balance. Emergency protocols in factories focus on spill prevention and quick cleanup to keep accidental releases in check.
Future Prospects
Looking ahead, demand for ammonium dihydrogen phosphate grows alongside population and the need for higher crop yields. Sustainable manufacturing practices will call for lower-carbon input processes, recycling of byproducts, and real-time purification checks. Companies investigate biotechnological processes that leverage genetically engineered microbes for converting waste ammonia or phosphate into fertilizer-grade ADP. Automation often reduces dust in packaging lines, improving workplace safety. Out in the fields, precision agriculture tools—drones, remote sensors—already recommend how much and when to apply, reducing environmental impact and expense for growers. Researchers expect next-generation ADP blends to pair with digital farming for even more efficient, tailored nutrition.
What is Ammonium Dihydrogen Phosphate used for?
Boosting Crops from the Ground Up
Farming demands more than sunlight and water. Ammonium dihydrogen phosphate lands in the hands of growers because it blends two essentials: nitrogen and phosphorus. On my family’s farm, we watched the difference in plant growth when fertilizers had the nutrients balanced just right. Nitrogen gives crops a push in their leafy growth, and phosphorus helps roots dig deep. Fields of corn or wheat, treated with this compound, come up fuller and healthier, especially in soils missing these nutrients. The yields climb, but there’s another side: too much, for too long, causes runoff, which harms rivers and drinking water. It’s not about spreading as much as possible—all growers need to keep an eye on soil tests, seasons, and rainfall. Responsible use starts with knowing what your ground lacks.
Fire Safety in Everyday Places
Many folks don’t realize that ammonium dihydrogen phosphate shows up in the red powder inside fire extinguishers. At home, cooking accidents or electrical shorts do more than make a mess. This powder plays a big role in slowing down or putting out the flames before they can turn small accidents into loss. It forms a layer over hot surfaces, stopping oxygen from fueling the fire. I remember the difference a small extinguisher made in my neighbor’s garage—quick thinking and the right powder saved more than the tools. It’s hard to put a price on that peace of mind.
Cleaner Power, Brighter Screens
Outside farms and kitchens, this chemical steps into tech labs. Makers of semiconductors and electronics use it for its crystal-forming ability. Creating these tiny parts demands high purity and evenness—especially for LEDs and screens lighting up our homes and offices. Research labs rely on this ingredient to grow crystals for experiments in optics or new energy tech. Better crystals mean sharper screens for movie nights and smoother power flow in electric cars. Factories keep close watch on quality, knowing sloppy production costs millions, not just dollars but trust in innovation.
Teaching and Science in the Classroom
Kids remember the simple stuff they see and touch. Mixing ammonium dihydrogen phosphate in a classroom can form beautiful “Magic Crystals”—a favorite science demo. Getting students excited about chemistry doesn’t always start with big talk about atoms and ions. It starts when they mix a solution, wait a day, and then see sparkly results in a jar. Teachers use these experiments to give real meaning to the words “phosphate” and “ammonium”—building curiosity that beats out memorization. Plenty of science fairs still have crystal gardens on display, and for good reason.
Thinking Ahead: Balance is Everything
Across fields, factories, and homes, ammonium dihydrogen phosphate shows up more than people think. Its benefits are clear, but there’s a fine line between smart use and misuse. Over-fertilization or poor disposal can strain the land and water. Farmers can rotate crops and test soils, homeowners can check extinguisher dates, and manufacturers can invest in waste treatment and cleaner production. Everyone who uses products built on chemistry can push for smarter practices and a bit more care in daily choices. Progress depends on remembering both the upsides and the risks of what we rely on.
Is Ammonium Dihydrogen Phosphate safe to handle?
What This Stuff Actually Does
Ammonium dihydrogen phosphate pops up in more places than a lot of folks realize. In classrooms, jars with crystalline white powder form part of basic chemistry sets. Farmers use it, too, mixing it into soil for its rich supply of phosphorus and nitrogen. Firefighters know it well, sprinkling it over flames to starve fires. It’s not some rare, exotic chemical.
Personal Experience Meets the Lab Bench
Years back, I spent afternoons in a university lab, brewing up solutions for plant studies. We’d handle ammonium dihydrogen phosphate in plastic scoops before dissolving it in water. I remember the slight tangy scent, sort of like a whiff of minerals after rain. Touching the powder never burned or stung my skin, but lab gods always demanded gloves and goggles for a reason.
Unpacking the Safety Concerns
No chemical belongs in the kitchen cupboard next to flour, but ammonium dihydrogen phosphate rates fairly low on the hazard scale. It isn’t explosive or unpredictable. Skin contact won’t send you to the ER, and a few crystals won’t smoke like struck matches. Still, chemical safety sheets warn about eye irritation, nose tickles, and, for some, bouts of sneezing or coughing if the fine dust floats up.
More serious trouble comes with bigger spills. Lots of powder in one spot means risk of slippery floors, and breathing in clouds of dust daily can irritate lungs over time. Swallow a heap, and you’ll feel pretty rough — an upset stomach, vomiting, or diarrhea. Nobody wants that.
Backing Facts with Science
The Environmental Protection Agency lists ammonium dihydrogen phosphate as non-toxic to fish and low risk to soil organisms when used as a fertilizer. Its solubility in water helps it break down quickly. Large spills in rivers do affect water quality, but most everyday uses don’t reach that scale. The fire extinguisher versions often end up as harmless compounds after fighting flames.
Smart Habits, Less Risk
Busting out the blue nitrile gloves in the classroom or garden seems like overkill to some, but good habits stick. If a ten-year-old can learn to avoid rubbing eyes or inhaling powder, everyone else should catch on too. Chemical goggles guard against stray dust, and dust masks prevent a dry throat. Grown-ups using it for farming, science, or battling electrical fires wear personal protection as a matter of course. I’ve heard stories from seasoned farmhands who use a simple trick — never open bags upwind, and store the stuff away from curious kids.
Room for Improvement in Awareness
Clearer labels and bold instructions would help people use ammonium dihydrogen phosphate with fewer worries. It isn’t the most dangerous thing in the shed, but sharp warnings never hurt. Science teachers, parents, and crew chiefs should keep first-aid info handy, making sure everyone knows that plenty of water, open air, and quick thinking solve most slip-ups. If in doubt, treat the chemical with the same respect you’d give to bleach or ammonia — not dangerous in steady hands, but not worth playing around with.
What Safer Handling Looks Like
Better storage practices come from common sense. Keep containers sealed. Avoid mixing with strong bases or acids, since those get lively. Wash hands after use, wipe down work surfaces, and keep eating or drinking away from the action. Spills deserve prompt cleanup, never a sweep under the rug. Folks working in greenhouses or laboratories stay safer with regular reminders posted in plain sight. Real safety comes from habit, not just paperwork or warnings.
What is the chemical formula of Ammonium Dihydrogen Phosphate?
Breaking Down the Chemical Formula
Ammonium dihydrogen phosphate pops up in classrooms across the world during chemistry lessons. The chemical formula is simple: NH4H2PO4. It combines ammonium (NH4+) and dihydrogen phosphate (H2PO4-). That string of letters and numbers means more than just something out of a textbook. It helps farmers feed their crops, firefighters beat back wild blazes, and even young students grow crystals for science fairs.
A Fertilizer that Makes a Difference
Walking through a hardware store, you’ll likely spot bags labelled with big letters noting their N-P-K ratios—those stand for nitrogen, phosphorus, and potassium, three nutrients crops need to thrive. Ammonium dihydrogen phosphate shines because it delivers both nitrogen from the ammonium and phosphorus from the dihydrogen phosphate, at the same time. I spent time working with school gardens, mixing this fertilizer into the soil, watching the beans and squash respond almost overnight with a deeper green and sturdier growth.
Globally, over 150 million tonnes of phosphate fertilizers find their way into fields each year. Some people worry about runoff contaminating water, and they have a point. Fast-growing plants may help, but smart application, not dumping, means less runoff and healthier crops. Agricultural extension offices often run soil tests to guide applications, which helps keep levels in the soil balanced.
Stopping Fire in Its Tracks
Few people realize it, but ammonium dihydrogen phosphate forms part of the red slurry helicopters dump on wildfires. The compound releases water and ammonia when exposed to intense heat, which helps cool flames and coat surfaces. That buys time for firefighters. My uncle was a volunteer firefighter, and he talked about how fire retardant lines would sometimes be the only chance to protect homes or forests out West.
Some environmentalists point to risks from chemical use, though. Years of observation and field research helps improve application methods. Crews look for buffer zones to protect waterways. Regulatory agencies regularly update their practices based on new studies. These ongoing improvements let us keep using the compound where it counts, without causing unnecessary damage.
Learning from Lab Benches
Students often grow sparkling crystals using ammonium dihydrogen phosphate. In my science club, this experiment never failed to grab attention. The whole process teaches about solubility and crystallization. Textbooks can only take you so far; actually seeing those crystals form cements the lesson. Chemical formulas become something real—tangible, sharp, and fascinating.
Paths Forward
This compound’s usefulness runs wide. The challenge comes in keeping its benefits without stray impacts. Better soil testing, precise fertilizer placement, and careful firefighting tactics all add up. Ammonium dihydrogen phosphate can stay a force for food and safety. Nobody gets every move right—mistakes happen, and the world learns from them. Listening to experts and local communities, then adjusting approach, moves everybody in the right direction.
How should Ammonium Dihydrogen Phosphate be stored?
Recognizing the Risks and Priorities
Most people at some point rely on fertilizers or chemicals in farming, gardening, or manufacturing. Ammonium dihydrogen phosphate sits high on the list of popular and useful products due to its solid reputation as a reliable fertilizer, flame retardant, and even in electronics manufacturing. Yet, anything that earns such wide use needs a little extra care. Mishandling or poor storage can lead to wasted resources, health hazards, or even accidents, so it pays to take a minute and look at things from the ground level.
Why Storage is More Than Just Space on a Shelf
Walk into a supply shed or chemical storeroom and it’s clear some folks treat storage as an afterthought. Bags ripped open. Piles of powder mixed with fertilizer runoff. This is where small mistakes turn into big ones. Ammonium dihydrogen phosphate absorbs water right out of the air—a property chemists call hygroscopicity—so moisture is the enemy. Let the powder pull in water, and you get clumps, reduced effectiveness, and a headache when it finally comes time to use the product.
Temperature swings spell more trouble. Warm, humid air creeping into a storage room practically invites clumping and caking. Over time, these changes can break down the chemical structure, too. Those fine particles also have a way of floating everywhere, covering shelves, bags, and skin. Good practices don’t just protect product quality—they protect lungs, eyes, and safety every single day.
Safe Storage Begins with Awareness
Some folks take pride in a tidy barn, but chemical storage calls for a higher bar. Start with the basics—a cool, dry, and well-ventilated area. The “cool” part isn’t about luxury. Extra heat speeds up moisture absorption and decomposition. Direct sunlight heats up storage areas quickly and will do more harm than good. Stack bags or containers on pallets, not straight on the ground, to dodge sneaky leaks and accidental spills.
Keep water and damp gear far from your stash. I learned early on that storing even sealed bags below a leaking pipe or roof tile turns reliable fertilizer into a solid block that’s tough—sometimes even impossible—to use. Once, a batch of fertilizer stored in a damp corner hardened into something closer to concrete and had to be tossed completely.
Avoid Cross-Contamination and Accidents
Mixing up chemicals seems harmless until you see damaged sacks, mystery powders, and labels lost. Ammonium dihydrogen phosphate interacts badly with strong acids and oxidizers. Store it far from materials like bleach, lime, or fuels. Label everything clearly. Eye wash stations and gloves matter too—scrambling for protection in the middle of an accident wastes precious minutes.
Don’t just slap on a padlock and call it secure. Restricting access keeps out curious kids and pets, but it also means only trained staff handle the material. In a busy farm or garden center, training everyone on the right handling practices keeps both business and community safe.
Regular Checks Make a Difference
Shortcuts with record-keeping show up fast. Keep logs of all purchases and uses. Inspect containers often. If a bag looks damaged or product clumps up, fix the problem right away—don’t wait for the next person to stumble upon it. Getting in the habit of regular checks catches problems before they hurt your bottom line, health, or environment.
Simple Solutions Keep Things Safe
Managing ammonium dihydrogen phosphate is often about consistency, not complexity. Good ventilation, dry storage, and regular inspections go further than any high-end gadget or fancy container. Rely on common sense and lessons learned. A few minutes of care today beats a risky scramble later.
Is Ammonium Dihydrogen Phosphate soluble in water?
Looking Closer at This Useful Compound
Ammonium dihydrogen phosphate, often found under the label ADP, connects deeply to farming, fire safety, and even science classrooms. Most of us catch glimpses of it in fertilizer bags or fire extinguishers, but the real magic happens once it hits water. For many practical purposes, people care about how quickly and completely something dissolves. That’s where ADP stands out.
How It Behaves in Water
ADP dissolves easily in water. Scoop some white crystals into a beaker, stir, and you'll see it vanish without much fuss. The numbers back it up — over 350 grams slip into a liter of cold water, and the rate only climbs if you add heat. This means a glass of water transforms into a clear, ionic solution jam-packed with ammonium and phosphate ions, ready to do their work, whether in fertilizer or a lab reaction.
Why Farmers and Gardeners Care
Food doesn’t grow without healthy soil. Phosphorus fuels plant development and root strength, while nitrogen supports leafy green growth. ADP brings both to the table, but its real edge comes from its solubility. Quick-dissolving nutrients spread evenly once watered, giving roots a fair shot at absorbing them. This approach means fewer dead spots in the field and less time waiting for results. Anyone working the land knows that a water-soluble fertilizer works faster and causes less waste. It fits into drip systems and hydroponics without leaving behind muddy residue that can clog pipes or rot roots.
Safety and Scientific Use
In safety, ADP often serves as a backbone for dry chemical fire extinguishers found in kitchens and businesses. After discharging, water can easily wash away the powder, thanks again to its high solubility. In classrooms, teachers rely on it for crystal-growing experiments for students. Clean-up remains hassle-free, a real blessing for both educators and lab techs. The ability to dissolve it quickly also keeps experiments on schedule and prevents contamination between lab tests.
Challenges and Responsible Use
Yet, too much of a good thing can spell trouble. ADP’s ready solubility means any extra supply on fields can run off after rain. High phosphate levels in rivers can harm natural ecosystems, fueling algae blooms that choke out other aquatic life. Managing application and following agronomy guidelines keeps the focus on production without sidelining environmental responsibility. People in agriculture often test soil first before adding ADP, making sure what they apply matches real crop needs.
Room for Smarter Approaches
New technology is driving change. Controlled-release fertilizers, precision irrigation, and real-time monitoring help match what crops get to what they can actually use. For folks in other industries, better packaging and clear use instructions keep handling safe. Researchers keep looking for blends that dissolve well but don’t move as quickly through soil, supporting both crops and long-term land health.
Science at Work in Everyday Life
ADP’s solubility bridges science and living — feeding crops, fighting fires, and making lessons come alive. People using it see real results thanks to its fast action in water. That same property calls for mindful dosing and smart technology, so its benefits stick around without unexpected side effects.
| Names | |
| Preferred IUPAC name | azanium dihydrogen phosphate |
| Other names |
Ammonium phosphate monobasic Monoammonium phosphate MAP Ammonium dihydrogen orthophosphate Ammonium dihydrogenphosphate |
| Pronunciation | /əˈmoʊniəm daɪˈhaɪdrə(d)ʒən fəˈsfeɪt/ |
| Preferred IUPAC name | ammonium dihydrogen phosphate |
| Other names |
Monoammonium phosphate Ammonium phosphate monobasic Ammonium dihydrogenorthophosphate MAP |
| Pronunciation | /əˈməʊniəm daɪˈhaɪdrɪdʒən fəˈsfeɪt/ |
| Identifiers | |
| CAS Number | 7722-76-1 |
| Beilstein Reference | 1688732 |
| ChEBI | CHEBI:62957 |
| ChEMBL | CHEMBL1201633 |
| ChemSpider | 20814 |
| DrugBank | DB14440 |
| ECHA InfoCard | 03c246d8-62a3-422e-a1b1-e033d675bad6 |
| EC Number | 231-764-1 |
| Gmelin Reference | 1164 |
| KEGG | C01059 |
| MeSH | Dihydrogenphosphate of Ammonia |
| PubChem CID | 24857 |
| RTECS number | **BP3675000** |
| UNII | 7Z6XZW3W1E |
| UN number | UN2061 |
| CompTox Dashboard (EPA) | DTXSID2020822 |
| CAS Number | 7722-76-1 |
| Beilstein Reference | 358998 |
| ChEBI | CHEBI:63034 |
| ChEMBL | CHEMBL1201591 |
| ChemSpider | 11119 |
| DrugBank | DB14538 |
| ECHA InfoCard | 03c217e5-189a-4de7-9214-c6db0bcee4a2 |
| EC Number | 231-764-1 |
| Gmelin Reference | 12011 |
| KEGG | C01147 |
| MeSH | D015550 |
| PubChem CID | 24856 |
| RTECS number | BP4550000 |
| UNII | 7UED189YKT |
| UN number | UN2348 |
| CompTox Dashboard (EPA) | DTXSID2020637 |
| Properties | |
| Chemical formula | NH4H2PO4 |
| Molar mass | 115.03 g/mol |
| Appearance | White crystals or crystalline powder |
| Odor | Odorless |
| Density | 1.8 g/cm³ |
| Solubility in water | 368 g/L (25 °C) |
| log P | -2.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | Acidity (pKa) of Ammonium Dihydrogen Phosphate: "2.12 |
| Basicity (pKb) | 11.9 |
| Magnetic susceptibility (χ) | −62.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.527 |
| Dipole moment | 5.8 D |
| Chemical formula | NH4H2PO4 |
| Molar mass | 115.03 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.8 g/cm³ |
| Solubility in water | 575 g/L (25 °C) |
| log P | -3.8 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa ≈ 5.4 (for dihydrogen phosphate ion) |
| Basicity (pKb) | 11.9 |
| Magnetic susceptibility (χ) | −51.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.522 |
| Dipole moment | 2.32 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 93.3 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1288.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1366 kJ/mol |
| Std molar entropy (S⦵298) | 99.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −1528.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1347 kJ/mol |
| Pharmacology | |
| ATC code | S12CA02 |
| ATC code | V04CX01 |
| Hazards | |
| Main hazards | Eye, skin, and respiratory tract irritation. |
| GHS labelling | Not classified as hazardous according to GHS |
| Pictograms | GHS07 |
| Signal word | Non-hazardous |
| Hazard statements | Not a hazardous substance or mixture. |
| Precautionary statements | P234, P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | > 210 °C (410 °F) |
| Lethal dose or concentration | LD50 (Oral, Rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat) 5750 mg/kg |
| NIOSH | SN1225000 |
| PEL (Permissible) | 15 mg/m3 (total dust), 5 mg/m3 (respirable fraction) |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | No IDLH established |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | NFPA 704: 1-0-0 |
| Autoignition temperature | > 400 °C (752 °F; 673 K) |
| Lethal dose or concentration | LD50 (oral, rat): 5750 mg/kg |
| LD50 (median dose) | 5750 mg/kg (Rat, oral) |
| NIOSH | WS4250000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Monoammonium phosphate Diammonium phosphate Ammonium phosphate Phosphoric acid Ammonium sulfate |
| Related compounds |
Monoammonium phosphate Diammonium phosphate Ammonium phosphate Phosphoric acid Ammonium sulfate |

