Ammonium Bicarbonate: An Evolving Ingredient in Science and Industry
Historical Development
Ammonium bicarbonate earned its place in food and industry long ago. People in Europe first relied on it in the 17th and 18th centuries as “baker’s ammonia,” an ingredient that let baked goods puff up before modern baking powder. Bakers swore by it for its power to generate carbon dioxide, which gave cookies a crisp bite that baking soda couldn’t match. Factories then found value in its decomposing property, leading to widespread use in leavening, fire extinguishers, and early photography. Those successes set the stage for future innovation, pushing research toward cleaner and more efficient ways to create and apply ammonium bicarbonate on larger scales.
Product Overview
You’ll run into ammonium bicarbonate in a few familiar places. In kitchens, it shows up under old labels like “hartshorn salt” or “baker’s ammonia.” Industrial labels call it ammonium hydrogen carbonate. Each batch looks like a white, powdery solid, dissolving in water almost instantly and giving off the faint scent of ammonia. Most people see it as an additive for cookies and crackers, but chemical labs use it in reagents, and nitrogen fertilizer blends rely on it to boost plant growth. The versatility pushes companies to refine the powder’s purity and size for every application.
Physical & Chemical Properties
Pure ammonium bicarbonate doesn’t hide its character. With a formula of NH4HCO3, it breaks apart in water, releasing ammonia and carbon dioxide, and starts decomposing above 36°C. Anyone who’s worked in a kitchen with it remembers that telltale ammonia smell, especially when it heats up. High solubility in water but not much in alcohol sets it apart. This substance stands out for vanishing without a trace when roasted, leaving no solid residue in baked goods—a unique gift for bakers seeking a clean finish.
Technical Specifications & Labeling
Quality standards demand detail, especially on food-grade ammonium bicarbonate. Labels on European or U.S. batches reflect purity levels—over 99% most times—as well as limits for heavy metals, moisture, or chloride contamination. Safety placards focus on keeping it sealed, dry, and away from acids, since stray storage can end in pungent odors or hazardous fumes. Chinese and American food safety laws both require batch information, use instructions, and clear producer identification to foster trust with buyers and regulators.
Preparation Method
Production pulls from both tradition and efficiency. On an industrial scale, engineers bubble carbon dioxide and ammonia gas together in water under cool conditions, letting the compound slowly crystallize out. Operators filter, wash, and dry the powder, tweaking temperature and flow rates for the cleanest crystal formation. Smaller labs sometimes form it by adding ammonium carbonate to carbon dioxide, but large factories prefer gas-phase methods because of output consistency and cost control.
Chemical Reactions & Modifications
Working with ammonium bicarbonate sparks some classic reactions. Add acid and you get brisk bubbles—ammonia gas jets out. Heat it, and it vanishes to carbon dioxide, water, and ammonia, explaining its historical use as a leavening agent. Chemists can swap out some ammonia for other ammonium salts, yielding custom reaction paths for fertilizer or textile manufacturing. A few tweaks turn it from simple powder to functional additives in fire extinction or even protein extraction in life science labs.
Synonyms & Product Names
Supermarkets and chemical suppliers list ammonium bicarbonate under more names than people expect. Besides “baker’s ammonia” and “hartshorn,” technical listings include ammonium hydrogen carbonate and carbonic acid monoammonium salt. The food industry often prefers E503(ii), its EU code—small but meaningful for compliance. Each alias reflects a history shaped by culture and chemistry, so knowing the full list cuts confusion anywhere from the bakery to the bioprocessing line.
Safety & Operational Standards
Handling ammonium bicarbonate takes more than a casual approach. Anyone storing it learns quickly to keep it cool, dry, and tightly sealed. The powder pulls moisture and acid from the air, which triggers decomposition to ammonia—a real hazard in close quarters. OSHA and EU standards both warn workers to wear gloves, goggles, and face protection, and ventilation matters in production and labs, not just busy kitchens. Transport rules also restrict mixing it near oxidizers or acids. Emergency plans ask for ammonia leak control, spill cleanup, and safe disposal by water dilution—never landfill dumping. Regular training and updated hazard sheets maintain safety and environmental care.
Application Area
People know ammonium bicarbonate mostly from bakery recipes, but use goes much further. Agronomists pick it as a slow-release nitrogen source for crops, helping farmers feed more plants with fewer applications. Industrial users lean on it for foam fire extinguishers, where instant gas evolution matters most in suppressing flames. In life science labs, it acts as a buffer or reagent for protein sequencing. China’s food producers rely on its purity in millions of biscuits, while Germany and Scandinavia never dropped its place in traditional cookies.
Research & Development
R&D teams dive deep, searching for new ways to refine and improve ammonium bicarbonate. Food scientists pursue purer grades for gluten-free and allergen-free formulations, since trace residues in baked snacks spark consumer complaints. Environmental researchers test it as an eco-friendly fertilizer, measuring field results with modern sensors, while green chemistry circles probe into greener synthesis routes. Chemical engineers try to cut fossil input for feedstock, investigating bio-based ammonia streams or renewable CO₂ sources. Computational modeling unlocks new insights into reaction rates, optimizing production for lower waste. These moves support food security, industry efficiency, and sustainable manufacturing worldwide.
Toxicity Research
Ammonium bicarbonate offers both benefits and basic risks. Food safety reviews show that at standard concentrations in baked goods and fertilizers, toxicity for people and farm animals runs extremely low, since it decomposes fully to harmless gases at baking temperatures. Acute exposure to dust or gases brings irritant effects to eyes, noses, or lungs, pushing labs and plants to upgrade ventilation and training. Chronic high-dose testing in animals flags no severe systemic toxicity, but regulatory guidance still sets strict workplace and food limits. China and Europe each track “no observed adverse effect” levels under continuous review, focusing on vulnerable populations.
Future Prospects
Future demand looks strong for ammonium bicarbonate. Sustainable agriculture needs more slow-release nitrogen carriers that don’t damage soil, drawing attention back to traditional compounds. Clean-label trends in food push producers to revisit classic leaveners, and new baking techniques ask for purer, safer additives. Industry specialists look for greener, less energy-intensive synthesis, steering research toward renewable inputs. Expanded genomic and protein research in biotech uses more of it as a reagent, cementing its tool-kit status in labs worldwide. New regulations around fertilizer runoff and air quality will shape innovation, making safety, purity, and lifecycle analysis part of business planning from Europe to Asia.
What is ammonium bicarbonate used for?
The Pantry and the Bakery
Anyone who spends time in a kitchen or bakery has stumbled upon ammonium bicarbonate, even if they don’t realize it. People sometimes call it “baker’s ammonia.” Before baking powder showed up in the 19th century, this leavening agent did much of the heavy lifting. It helps cookies and crackers puff up, giving them their signature crunch and light texture. Bakers pick ammonium bicarbonate for recipes where you want lift without leaving a bitter aftertaste. When the cookie bakes, this powdery ingredient breaks down into carbon dioxide and ammonia gas, which makes dough rise fast and leaves behind a light, crisp texture — perfect for thin, dry treats. Families in Northern Europe often use it for traditional holiday sweets.
Food Processing and Convenience
This ingredient doesn’t just stay in small kitchens. Large-scale food processors lean on it for efficient, predictable results. The reason? Ammonium bicarbonate dissolves easily in water and disappears into the product, which means it doesn’t leave any strange flavor behind. Cereal, crackers, and even instant noodles use it for textural reasons. Sometimes food technologists want leavening power with a quick-dissolving punch, especially for products thin enough to allow the ammonia to escape while baking or frying.
Making Medicines and Treating Water
Doctors and pharmacists know ammonium bicarbonate isn’t just a kitchen curiosity. It pops up in certain compounds and can help buffer pH in pharmaceutical manufacturing. Some tablet mixes need the carbon dioxide gas from the breakdown for proper structure. On the water treatment side, plants may use it to control pH in municipal or industrial facilities. Balanced pH means safer water for drinking and industrial purposes. It’s efficient, easy to handle, and breaks down cleanly, making it a smart choice for operators who need reliable adjustments without side effects from additives.
A Role in Agriculture
Farmers and gardeners see ammonium bicarbonate as a simple fertilizer. It boosts nitrogen levels, which plants crave during stages of strong leaf or stem growth. Nitrogen is a core nutrient for most foods we eat, from wheat to leaf lettuce, and top-dressing soil with this chemical can punch up yields. Still, care matters. Too much can stress plants and upset the soil’s chemistry. Timing and responsible use keep everything working smoothly.
Everyday Chemistry Labs
Ammonium bicarbonate shows up in high school and undergraduate chemistry labs for experiments on gas production and reactions. Its tendency to break down into gases makes it a handy teaching tool. Students remember fizzy test tubes and the classic “smelling salts” aroma. Simple, recognizable compounds like this one make science approachable and practical, helping the next generation learn how things work beyond the textbook.
Looking at the Risks
No chemical solves every problem by itself. Ammonium bicarbonate earns trust because it breaks down into harmless gases and leaves no toxic residues when used right. But there’s a catch: in closed spaces, ammonia fumes build up and can burn the eyes and nose, which is why bakery kitchens and factories monitor ventilation. Some people also react to ammonia traces in food. Learning to use the right dose and airing out workrooms helps keep workers and families safe.
Smarter Use Starts with Awareness
Knowing how and when to use ammonium bicarbonate means reaching for reliable science and local best practices. Tighter controls around industrial kitchens are pushing for smarter dosing and better ventilation, keeping both products and people in good shape. Farms benefit by checking soil chemistry before dumping anything new onto their fields. The more we learn from past mistakes and success stories, the more we create a food and farming system that safely uses what chemistry has to offer.
Is ammonium bicarbonate safe to eat?
Understanding What Ammonium Bicarbonate Does
Ammonium bicarbonate pops up on a lot of food labels, and many people never give it a second thought. Bakers might have noticed it on the label of baking powder, especially if you grew up seeing your grandmother reach for that faded white can in the cabinet. The compound works much like baking soda, but it acts even faster. In recipes, it helps doughs rise by releasing gas as soon as moisture and heat hit it.
Tracing Its Safety in the Kitchen
Government agencies, including the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority, have reviewed ammonium bicarbonate and put it on the list of food additives considered safe, as long as you’re not throwing back massive spoonfuls daily. The quantities used in cookies or crackers sit far below anything that would cause trouble.
Grandparents used it to make traditional German and Scandinavian holiday cookies like Springerle or Lebkuchen. The compound's quick-release reaction gives old-school recipes their light texture, something other leaveners can’t quite reproduce. Even chefs with decades in the business rely on it for that unmistakable crispness.
The Science Behind Its Use
When ammonium bicarbonate hits hot dough, it breaks down into ammonia, carbon dioxide, and water. The carbon dioxide gives baked treats their lift. Most people never taste or smell the ammonia, because the heat in a proper bake drives those vapors out of the kitchen long before anything gets served. That’s why bakers emphasize thin dough and enough oven time, especially since thicker goodies can sometimes trap the sharp scent.
Honestly, anyone who’s ever eaten a crispy cracker, a light meringue, or a thin wafer has probably had a tiny bit of this additive. The food industry leans on it for texture, not taste, because it leaves no trace after baking. Even so, some folks avoid it out of worry about possible ammonia release. Yet ammonia already exists in small amounts in meats, cheeses, and even our own bodies. The amounts in a kid’s birthday cookie are much lower than what we encounter in daily life.
Health Perspectives and Concerns
Concerns often circle around the word “ammonia” on ingredient labels. People picture the household cleaning product and imagine risks much bigger than the facts support. The ammonia produced during baking escapes before the food even gets plated, as long as recipes and baking guidance are followed. For people with lung conditions or certain allergies, any airborne irritant can raise questions, but these cases remain rare, and most folks never notice a difference.
No evidence links eating ammonium bicarbonate in baked goods with cancer, asthma, or neurological issues. The FDA lists it as GRAS (Generally Recognized as Safe). Toxicologists note the body can process small bits of ammonia, whether it’s from food or the air. There’s always room for allergies or sensitivities when dealing with anything added to food, but ammonium bicarbonate doesn’t stand apart from common salt or baking powder in that sense.
Where to Pay Attention
Some home bakers and anyone curious can err on the side of caution by keeping baked goods thin and giving them plenty of time in the oven. Large-scale food producers already monitor ingredient levels based on regulatory advice. Eliminating unnecessary additives from our diets makes sense, but ammonium bicarbonate isn’t a major culprit when it comes to risk.
Everyone deserves a safe plate of food. Knowledge, a bit of tradition, and a lot of science all show ammonium bicarbonate checking out for use in the kitchen, especially for those treats where nothing else gives quite the same result.
How should ammonium bicarbonate be stored?
Understanding What’s At Stake
Every so often, the basics matter more than the bells and whistles. Storage looks simple. Sitting there on a shelf, a white crystalline bag marked “ammonium bicarbonate” doesn’t seem like much trouble. The thing is, too many folks underestimate what careless storage can cause—especially with something that breaks down quickly in the wrong spot. There’s more to safety than putting a bag behind a locked door.
Moisture: The Quiet Thief
Humidity loves ammonium bicarbonate. Cloudy air spells trouble here. The moment moisture gets in, the product may start breaking down. Keeping it away from water vapor is not about paranoia, it’s about doing what works in the real world. I once watched an entire shipment turn into a clumpy, useless mess from just a few small tears in a sack, all because someone figured a little dampness wouldn’t hurt. It cost a lot in lost product and extra cleanup.
Dry, well-ventilated storage areas beat fancy systems. Use thick, sealed containers. Double bagging might look old-fashioned, but it works. Some stores set up simple dehumidifiers near their stock; others place silica gel packs inside bins. These aren’t high-tech steps, but they save money and cut waste. Anyone who’s lost a bag to humidity learns fast—don’t gamble on dry spells lasting forever.
Keeping Cool: Avoiding Risk
High temperatures push ammonium bicarbonate to decompose and produce ammonia gas. The smell hits first, then irritation in the eyes and nose. In one bakery, poor ventilation and storage above the oven turned a quiet corner into a hazmat scare. No one likes the cost of air circulation gear or extra insulation, but it sure beats a shut-down after a health scare.
If storage sits near heat sources, the risk just grows. Stash the product in the coolest area on site, far from boilers, ovens, or sun-facing windows. Even storing it near an upper shelf, away from steam lines or rooftop sunlight, helps keep the chemical stable. Keeping it cool isn’t just a guideline. It protects people in the warehouse, kitchen, and lab.
Labels, Spills, and Safety Lessons
Good labels make a difference. Too many places leave unlabeled bags lying around, counting on memory. Labels with purchase dates cut confusion. Marking the hazard keeps new staff aware that this isn’t table sugar. Sometimes, accidents come from complacency, not bad luck. Every employee should know to sweep spills quickly, and not to use water—that only makes the gas issue worse.
Personal protective equipment resists being forgotten if it sits near the storage bins. Gloves, goggles, and masks shouldn’t hide in a manager’s locked office. Putting basic instructions on the wall, where staff unload, saves time chasing down a supervisor if something goes wrong. Safe storage isn’t all about chemicals. It’s about respecting people’s health, paychecks, and time.
Better Habits Outperform Rules
No written rule covers every accident, but habits shaped by respect keep dangerous surprises away. I’ve watched what happens when people ignore the little stuff and pay the price—product loss, wasted hours, an ER visit. Safe storage, done right, protects a lot more than a store’s bottom line.
What are the alternatives to ammonium bicarbonate in baking?
Why Bakers Look Beyond Ammonium Bicarbonate
Ammonium bicarbonate has earned its place in old-school European baking, lending a crisp texture and lightness to recipes like gingerbread or certain flat cookies. Still, plenty of bakers and home cooks end up searching for alternatives. Good reasons back this up – ammonium bicarbonate releases ammonia gas during baking, something you can taste and smell if the baked goods are too thick or underbaked. It’s not always easy to find in stores, either. For anyone with sensitive stomachs or those after a more neutral flavor, switching things up can lead to better results and happier eaters.
Baking Powder Steps In
Baking powder shows up in kitchens around the globe because it does the job well for most cakes, muffins, cookies, and quick breads. This common leavening agent combines an acid and a base, so it generates carbon dioxide as soon as it gets wet and then again in the oven when it heats up. In my own kitchen, I’ve swapped ammonium bicarbonate for baking powder in thin, spiced cookies, and they still came out crisp and light. If the recipe calls for one teaspoon of ammonium bicarbonate, reaching for a teaspoon of baking powder usually works fine, but sometimes there’s a small drop in crispness.
Baking Soda’s Role
Baking soda gives another option, especially for recipes that use an acidic ingredient like buttermilk, yogurt, or cream of tartar. The acid jumpstarts the reaction, sending carbon dioxide through the dough or batter. For anyone aiming to avoid ammonia’s aftertaste, baking soda offers predictability and a clean flavor profile. I’ve used it in Scandinavian-style cookies, relying on lemon juice or sour milk to get that rise just right.
Yeast for Traditionalists
Yeast takes patience, but for certain breads or pastries, it produces depth in flavor no other leavener matches. Ammonium bicarbonate never delivers the slightly tangy, old-world taste that yeast can. Many classic recipes—soft pretzels, some flatbreads—come to life with slow yeast fermentation. Long-rising yeast dough also develops a stronger, more pleasing structure compared to chemical leaveners.
Self-Rising Flour for Baking Shortcuts
In busy kitchens, self-rising flour can help. It’s just regular flour blended with baking powder and salt. Some home bakers rely on it for biscuits, pancakes, or certain quick breads. Swapping a cup of all-purpose flour and leavener for self-rising flour leads to dependable results, provided you already control the salt content of your overall recipe.
Making the Right Choice for Each Recipe
Each of these substitutes brings unique benefits and potential quirks. Baking powder and baking soda lack the punchy crispness that ammonium bicarbonate gives to wafer-thin cookies, but they offer a safer and more convenient experience. Yeast requires extra time. Even so, modern bakers get steady, repeatable results from these everyday tools. Food safety matters too—nobody wants their cookies to send off clouds of ammonia if they’re a little underbaked. Instead, using alternatives lets bakers control both quality and taste, protecting the experience of sharing food with friends and family.
Improving the Process and Reducing Waste
Switching to accessible leaveners like baking powder and soda means less searching specialized stores or online suppliers. Less food ends up wasted since recipes prove less sensitive to being slightly undercooked, and everyone can enjoy the final product. These options also meet dietary needs for many people, since some find ammonium compounds harsh on their stomach.
Final Thoughts on Tradition and Change
Some traditional recipes still taste best with ammonium bicarbonate—it’s hard to fully replicate certain European cookies without it—but for almost everything else, regular baking powder or soda meets the needs of today’s home and professional kitchens. Confidence comes from knowing your substitutes well, testing them, and using what fits your style and your guest’s tastes.
Can ammonium bicarbonate be used as a cleaning agent?
What Makes Ammonium Bicarbonate Stand Out?
People usually spot ammonium bicarbonate sitting in the baking aisle, branded as baking ammonia or baker’s ammonia. Not everyone knows it has roots in cleaning. In the past, this white powder showed up in recipes, but it also found occasional use in household chores. Its strong ammonia scent made it handy for tackling certain stubborn spots, similar to how people use plain ammonia water. Once it breaks down, ammonium bicarbonate produces ammonia gas, water, and carbon dioxide—each with its own cleaning qualities.
Looking at the Science and the Risks
Ammonia-based cleaners remain popular for a reason. Ammonium bicarbonate fits roughly in this family, but with a twist: it’s milder than grocery-store cleaning ammonia. Add it to water, it starts to give off ammonia — nowhere near as potent, but enough to help cut through grease or grime on hard surfaces. This reaction, though, needs warmth and a bit of patience, since ammonium bicarbonate works best in a solution, not as a dry powder.
On the other hand, the serious ammonia smell can sting the nose or even irritate airways, especially in smaller, closed rooms. Keeping windows open helps, just as it would with regular ammonia cleaners. Ammonium bicarbonate should absolutely stay out of reach from little kids and pets—it tastes bitter, but small hands don’t always care what something smells like. In my own kitchen, any cleaning product that produces noticeable fumes gets stored on the top shelf.
Benefits and Drawbacks in Everyday Cleaning
Scrubbing a stovetop packed with burnt-on food, a sprinkle of ammonium bicarbonate mixed into warm water lifts off some of the grease, especially if left to sit for a few minutes. It breaks down organic gunk, so coffee and tea stains don’t stand much chance either. Over the years, I’ve seen it bring shine back to old mugs and white countertops. People who prefer cleaning with simple, single ingredients sometimes like ammonium bicarbonate as it’s not loaded down with synthetic perfumes or dyes.
The flip side: you don’t see ammonium bicarbonate popping up on shelves beside the big-brand cleaners, and there’s a reason. Ammonia smells linger, and sensitive noses might complain. It won’t tackle mold or mildew the way bleach does, and it’s not a disinfectant. Glass surfaces like mirrors tend to cloud up after a wash unless rinsed carefully. If skin comes in prolonged contact with a strong solution, a rash or dry patches could pop up. Gloves handle the problem easily, but not everyone thinks to grab a pair before cleaning.
Better Choices and Practical Solutions
Stores now carry plenty of safer, specialized products for each job, some of them plant-based and fragrance-free. Baking soda and vinegar sit high on the list for eco-conscious cleaning. Both run double-duty as deodorizers and mild abrasives, and rarely pose an inhalation problem. For anyone wanting to skip big brand products, these alternatives land closer to the sweet spot of safety and effectiveness. Ammonium bicarbonate can work in a pinch, but only with good air flow, careful handling, and plenty of rinsing.
Supporting Safe Cleaning at Home
Most households looking to freshen up their routine can stick with baking soda, vinegar, dish soap, or citric acid. If ammonium bicarbonate still ends up on your shelf, label it clearly. Don’t mix it with strong acids, since that boosts fumes. Everyone who shares living space—children, the elderly, or pets—can breathe easier when all cleaning products are kept safe and straightforward. Safety and clear labeling shouldn’t feel like an afterthought.
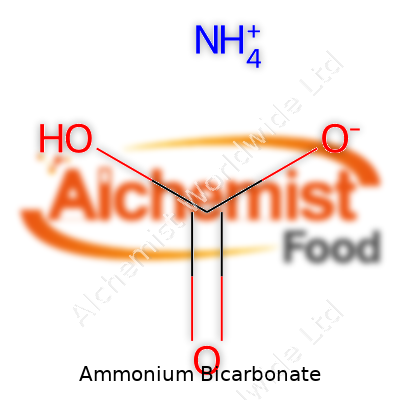
| Names | |
| Preferred IUPAC name | Ammonium hydrogen carbonate |
| Other names |
Ammonium hydrogen carbonate Bicarbonate of ammonia Hartshorn Ammonium acid carbonate |
| Pronunciation | /əˌməʊniəm baɪˈkɑːbəneɪt/ |
| Preferred IUPAC name | Ammonium hydrogen carbonate |
| Other names |
Ammonium hydrogen carbonate Bicarbonate of ammonia ABC Hartshorn Ammonium acid carbonate |
| Pronunciation | /əˌmoʊniəm baɪˈkɑːbənət/ |
| Identifiers | |
| CAS Number | 1066-33-7 |
| Beilstein Reference | 3587150 |
| ChEBI | CHEBI:62947 |
| ChEMBL | CHEMBL1082888 |
| ChemSpider | 5798 |
| DrugBank | DB04322 |
| ECHA InfoCard | ECHA InfoCard: 027-002-00-5 |
| EC Number | 205-633-8 |
| Gmelin Reference | 3770 |
| KEGG | C06321 |
| MeSH | D000648 |
| PubChem CID | 14013 |
| RTECS number | EB2980000 |
| UNII | ZY06NW0BRT |
| UN number | UN3077 |
| CAS Number | 1066-33-7 |
| Beilstein Reference | 4104221 |
| ChEBI | CHEBI:62957 |
| ChEMBL | CHEMBL1356 |
| ChemSpider | 5790 |
| DrugBank | DB04323 |
| ECHA InfoCard | 100.043.275 |
| EC Number | 205-633-8 |
| Gmelin Reference | 137941 |
| KEGG | C01342 |
| MeSH | D000648 |
| PubChem CID | 14013 |
| RTECS number | AB1925000 |
| UNII | G4SFW47U7E |
| UN number | UN3077 |
| Properties | |
| Chemical formula | NH4HCO3 |
| Molar mass | 79.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Ammonia odor |
| Density | 1.59 g/cm³ |
| Solubility in water | 24 g/100 mL (20 °C) |
| log P | -3.6 |
| Vapor pressure | Very low |
| Acidity (pKa) | 15.4 |
| Basicity (pKb) | pKb ≈ 3.77 |
| Magnetic susceptibility (χ) | −36.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.398 |
| Dipole moment | 5.1 D |
| Chemical formula | NH4HCO3 |
| Molar mass | 79.056 g/mol |
| Appearance | White crystalline powder |
| Odor | Ammonia-like |
| Density | 1.59 g/cm³ |
| Solubility in water | 22 g/100 mL (20 °C) |
| log P | -4.7 |
| Vapor pressure | Low (decomposes) |
| Acidity (pKa) | 15.4 |
| Basicity (pKb) | 3.77 |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ): −56.6 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.385 |
| Dipole moment | 4.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 216.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −897.3 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -871.2 kJ/mol |
| Std molar entropy (S⦵298) | 216.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -807.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −948.7 kJ/mol |
| Pharmacology | |
| ATC code | A12CO2 |
| ATC code | A12CO2 |
| Hazards | |
| Main hazards | May cause respiratory irritation, skin and eye irritation. |
| GHS labelling | GHS07, Warning, H319, P264, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation |
| Precautionary statements | P210, P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P337+P313, P362+P364, P403+P233, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | Ammomium bicarbonate does not have a specific autoignition temperature as it decomposes before reaching ignition. |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 (oral, rat): 1576 mg/kg |
| LD50 (median dose) | 2,130 mg/kg (rat, oral) |
| NIOSH | **SA9145000** |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 30 mg/m³ |
| IDLH (Immediate danger) | 500 mg/m3 |
| Main hazards | May be harmful if swallowed, inhaled, or absorbed through skin; causes irritation to skin, eyes, and respiratory tract. |
| GHS labelling | **"Warning, H319, Causes serious eye irritation, P264, P280, P305+P351+P338, P337+P313"** |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 oral rat 1300 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 2,130 mg/kg |
| NIOSH | SA9125000 |
| PEL (Permissible) | 50 mg/m³ |
| REL (Recommended) | 30 mg/m³ |
| IDLH (Immediate danger) | 500 mg/m3 |
| Related compounds | |
| Related compounds |
Ammonium carbonate Ammonium chloride Sodium bicarbonate Potassium bicarbonate |
| Related compounds |
Ammonium carbonate Ammonium carbamate Sodium bicarbonate Potassium bicarbonate |

