Aluminium Potassium Sulfate: An In-Depth Commentary
Historical Development
For centuries, people knew alum, or aluminium potassium sulfate, without understanding its full chemical structure. Ancient people in Egypt and Rome already valued it, not just for purifying water but for fixing dyes in textiles. During the Middle Ages, textile guilds and leather tanners paid high prices for it because rich colors stuck best with alum. The scientific study of alum picked up speed in the 18th and 19th centuries, once advances in chemistry revealed its make-up as KAl(SO4)2·12H2O. Modern-day alum production grew from these roots, as large-scale extraction from minerals like alunite and bauxite made it both commonplace and inexpensive. The historical journey of aluminium potassium sulfate sits at the crossroads of chemistry, commerce, and tradition.
Product Overview
Aluminium potassium sulfate pops up mostly as a crystalline solid—usually colorless, sometimes tinted with a pale white sheen. Its stoichiometric formula, KAl(SO4)2·12H2O, lands it in the broader family of double salts. Manufacturers sell it in lumps, powder, or blocks, depending on what clients need. In food processing, alum finds a place as a pickling agent because it keeps produce crisp, and it also turns up as a mordant in dyeing thanks to its locking-in abilities with fibers and pigments. In water treatment plants, workers add alum to pull tiny particles out of drinking water, a process with a huge impact on public health. Throughout the globe, millions of people rely on alum—even though they might not know its chemical name.
Physical and Chemical Properties
Alum holds water in its crystal structure, giving it a molecular weight just shy of 475 grams per mole. Its melting point runs around 92 °C, at which point it loses structural water, and heating further makes it break apart into fine alumina and mixed potassium-aluminium compounds. The solid dissolves easily in water, especially with added heat, forming a transparent solution. Touching alum, you might find it has a slightly astringent feel on the tongue. Chemically, it's a mild acid; its pH lands just below 4 in aqueous solution, which matters for its use in dyeing, as acidity helps certain colors bind fast to cloth. Unlike many salts, alum doesn’t release dangerous fumes and sits stable under normal conditions, which means it stores well for years with no special care.
Technical Specifications & Labeling
Industry buyers look at purity, as impurities—especially iron—can ruin batch processing in textiles or food. Most technical-grade alum runs above 99% purity, with loss-of-ignition tests checking for correct hydration levels. Standard labeling lists batch numbers, chemical name (both the IUPAC and common forms), net and gross weights, production date, country of origin, and a hazard pictogram if regulations call for it. Food-grade material comes with extra records, such as allergen statements and E number (E522 in EU regulations). Certificate of Analysis (CoA) reports go with every order above a certain tonnage, showing results for heavy metals, arsenic, and lead. For workers’ safety, Safety Data Sheets highlight the need for gloves and eye protection, though the compound carries a low hazard profile.
Preparation Method
Classic alum production begins with the roasting of alunite or bauxite ores to prep raw alumina. Manufacturers add potassium sulfate and run the mix through a series of dissolving, filtering, and crystallizing steps. The old “double decomposition” method still works: bring together solutions of potassium sulfate and aluminium sulfate, let them cool, and white alum crystals drop out. Industrial-scale plants recycle solutions and mother liquors to save energy and water. Careful pH control matters, as acidic conditions favor crystal growth. Today's operators often use centrifuges and vacuum filtration to get water-free crystals, then pass the product through drying tunnels so it holds the right water-of-crystallization. Finished product ships in lined bags or fiber drums, made to last against humidity and ambient CO2.
Chemical Reactions & Modifications
Alum doesn't react quickly at room temperature, but in the lab, it pairs with alkalis to make insoluble aluminium hydroxide gels. These reactions matter in water treatment plants where “floc” formation clears up turbid water. Add heat, and alum decomposes, first losing water, then breaking up into potassium sulfate, alumina (Al2O3), and sulfur dioxide. In dyeing, alum teams up with textile fibers to form coordinate bonds, helping colors stick in place. Modifying alum—by swapping potassium for ammonium or sodium—builds a big family of related salts, each with their own uses. Chemists today tweak alum for nanoparticles or slow-release fertilization, a field that keeps growing as precision agriculture spreads.
Synonyms & Product Names
Across languages and industries, alum goes by a list of names. Old merchants called it “potash alum” or “fitkari” in India. The pharmaceutical industry labeled it “alumen” in pharmacopoeias. In food tech, it answers to E522 or INS522. Even in geology labs, it turns up as “pearl alum” or “chrome alum” (for its chromium variant). Each name carries local, historical, or regulatory context, but the chemical fingerprint stays the same: one part potassium, one part aluminium, two parts sulfate, and a large amount of bound water. For exports, regulatory codes follow the Harmonized System (HS 283330), making cross-border trade simple for customs.
Safety & Operational Standards
Anyone handling alum works with a compound rated as an irritant but not a poison. Dust in the air can sting eyes and nose, so industrial plants vent workspaces and use dust collectors. Gloves and goggles stay on hand for employees bagging powder or cleaning filters. In food operations, the U.S. Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) cap daily intake for people at just 0.5 mg per kg body weight, guarding against long-term burden on the nervous system and kidneys. Wastewater rules ban dumping concentrated alum into municipal sewers; unused product gets disposed as non-hazardous solid waste, except in giant spills where pH swings could matter. Keeping the product dry in storage reduces both clumping and unwanted chemical changes. Training covers first aid for minor exposure—a rinse with water does the trick in most cases.
Application Area
Alum stands out for its versatility. Textile houses rely on it for bright dyes and lasting colors, with vinegar as the only real competitor for certain shades. Municipal water treatment plants owe their clear drinking water to alum’s knack for coagulating tiny particles, meeting World Health Organization (WHO) turbidity ratings without expensive technology. In food, chefs reach for alum to firm up cucumbers and watermelon rinds for pickling—though food safety laws limit its use. Pharmacies sell it to ease shaving nicks or as a base in deodorant blocks. Tanners use it in prepping animal hides, making the product key in both high tech and low tech cultures. Even schools keep alum for crystals in science fair experiments. Modern research explores its niche in slow-release fertilizer coatings and vaccine adjuvants, riding new waves of market demand.
Research & Development
Scientists dig into the structure–property relationship of alum all the time. Teams decode how cations swap and the way hydration shifts under pressure, trying to build designer salts for battery electrolytes or catalysts. Recent years saw a jump in nanoparticle research, where alum provides a low-cost, biodegradable shell for drugs or agrochemicals. Pharmacy labs test alum-based gels for wound care, banking on its astringent and antimicrobial qualities. Environmental engineers study modified alum, hoping it can suck up phosphate from lake water and stop harmful algal blooms. In biomedical labs, alum sees a role in vaccine formulations, acting as an adjuvant to boost immune response safely—though researchers hunt for next-generation versions to lift both safety and speed of action. Each field pulls alum in a new direction.
Toxicity Research
Exposure studies show most people tolerate alum at low levels, but scientists keep raising red flags about chronic use. Consuming high doses by mouth can hit the gastrointestinal tract with cramps and nausea. Animal research lists kidney stress above 50 mg/kg, which tells food processors to stay well below those levels. Sensitive groups—especially infants or people with kidney trouble—absorb aluminium more slowly, so food regulators enforce cut-off points and urge “least necessary” use in recipes. Scientists still debate the compound’s long-term links to neurodegenerative diseases. Animal studies suggest a tiny fraction of absorbed aluminium gets lodged in bone and brain, but human epidemiology paints a less clear picture. This scientific uncertainty pushes regulators to keep re-examining safe exposure levels.
Future Prospects
Looking forward, alum faces both opportunity and challenge. Water treatment will call for more of it as urban populations swell, though new techniques may compete for market share. In agriculture, demand appears ready to grow thanks to slow-release fertilizer coatings and environmental remediation. In health and medicine, research on alum's adjuvant power in vaccines could unlock better shots for hard-to-immunize populations. At the same time, pressure from green chemistry and calls to lower aluminium residues in everyday life spark a race for lower dosing, biodegradable alternatives, and smarter process controls. As industries change, alum’s role will shift from old-school chemistry to new arenas, shaped by regulations, public demand, and continuing advances in materials science.
What is Aluminium Potassium Sulfate used for?
Understanding the Role in Food
Aluminium potassium sulfate, often called potash alum or just alum, plays a small but vital part in many kitchens and food factories. Home picklers recognize it as the classic crisping agent for cucumbers and other vegetables. Adding a pinch helps firm up the texture, giving pickles their famous snap. Spend some time looking through ingredient labels at the store and you'll spot it in some baking powders, too. Here it takes the job of helping doughs and batters rise, offering an alternative to sodium-based additives.
The US Food and Drug Administration lists alum as generally recognized as safe (GRAS) for direct use in food, though it recommends limits. At home, most people don’t use much, sticking to the old practice of rinsing produce after an alum soak, just as grandma used to. Keeping that habit matters, because excessive alum can leave a trace of bitterness and isn't meant for big doses.
Alum in Water Purification
Walk into any municipal water treatment plant and you’ll likely find alum playing a starring part. City engineers add it to water supplies to help remove dirt and other particles. The chemistry here stays simple: alum reacts with impurities, causing them to clump together and settle out. This process, known as flocculation, produces cleaner drinking water for millions of people. In my experience volunteering with a rural water project, alum brought cloudy well water to drinkable clarity with surprising efficiency. The World Health Organization points to alum as a reliable method, especially in areas with limited options.
Alum’s Unseen Hand in Everyday Products
Outside the kitchen, the compound turns up in surprising places. Textile workers value it as a mordant in dyeing. Alum fixes dye to fabrics, making colors brighter and more durable. Next time you admire a vivid cotton scarf or a boldly patterned tablecloth, there’s a good chance alum played a role in keeping those colors from fading after a wash.
Some personal care products draw on alum’s astringent qualities. Certain aftershaves and natural deodorant stones use alum to help control minor bleeding or reduce bacteria. I remember my grandfather using an alum block after his morning shave, swearing by its sting to stop the odd nick.
Health and Safety Questions
People sometimes ask whether repeated exposure to alum poses risks. Research suggests that casual topical or culinary use isn’t a cause for worry, but large or regular ingestion should be avoided. European food agencies have pushed for stricter controls, especially when it comes to processed cheese and baked goods, since young children can be extra sensitive.
In medicine, doctors and dentists sometimes rely on alum to stop minor bleeding during minor surgeries or teeth extractions. The compound acts fast, reducing blood loss. This targeted use, not for swallowing, has stood the test of time. All the same, health experts encourage reading labels and following directions, sticking with well-established limits.
Looking for Balance
The story of alum shows how an old compound still finds its place in modern kitchens, water plants, and medicine cabinets. Knowing where it turns up helps consumers make informed choices. Industry groups and regulators keep an eye on how alum gets used, urging safe handling. People at home already practice common sense by measuring carefully and rinsing food well.
The key lesson for anyone is to stay aware, ask questions about unfamiliar ingredients, and look for updated safety advice. That open attitude keeps both tradition and well-being in balance—from the garden pickles, to clean water, to the next home remedy for a scraped knee.
Is Aluminium Potassium Sulfate safe for consumption?
Looking at Kitchen Staples: What’s in Alum?
Aluminium potassium sulfate, better known as alum, often shows up in spice racks and old-school pickling recipes. I remember the first time I saw my grandmother pull out a mysterious white powder during her annual cucumber pickling marathon. She’d explain that alum kept pickles crisp, so they snapped between your teeth. But over the years, questions about the safety of this time-honored ingredient have bubbled up, partly because aluminum gets a lot of headlines when it comes to health.
Regulations and Reality: What Experts Say
Authorities like the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) have both weighed in. The FDA calls alum "generally recognized as safe" when used in accordance with good manufacturing practices. Both agencies focus on level and frequency. Alum used in pickling arrives in tiny doses; a teaspoon or less per batch, and much of it washes away with brine before the pickles go in your mouth.
Studies examine how much aluminum our bodies can handle. The EFSA points to a tolerable weekly intake of 1 mg per kilogram of body weight. Most people get far less than this from food, even when they eat pickled veggies now and then. For context, aluminum also comes from other sources—processed cheese, baking powder, some antacids. Dietary exposure, assuming a balanced diet, stays low for most people.
Health Concerns: Separating Fact from Fear
People worry about aluminum because of its possible connection to Alzheimer’s disease and other neurological conditions. Decades of research hasn’t found clear evidence linking dietary aluminum to these illnesses. The brain’s natural barriers filter out most aluminum, and that includes the little bit in alum-treated foods.
Occasionally, bad outcomes pop up, but nearly always in industrial settings, or from taking large doses of aluminum-based medicines over years, not from home pickling. You’d have to eat an unreasonable amount of alum-preserved food to load your system with harmful metal levels. I know families who have passed pickle recipes down the generations without a rash of memory loss, which matches what experts report.
Children and Sensitive Groups
Kiddos and people with kidney issues process metals more slowly, so I keep an eye on what ends up in their lunchboxes. For families with these risks, it makes sense to limit foods high in aluminum—including antacids, not just pickles. Doctors sometimes suggest skipping alum in pickling, just as a safety cushion for vulnerable family members.
Practical Solutions in the Kitchen
If you want to avoid alum, the good news is that alternatives exist. Grape leaves, oak leaves, and black tea all offer natural tannins that keep pickles crisp. I’ve used grape leaves from the backyard; they work just fine, and add zero aluminum.
For commercial producers, proper labeling and portion guidance ensure consumers don’t overdo it. Home cooks can read up or consult health professionals if they’re ever unsure. Knowledge—not just habit—should guide ingredient choices. Today’s science shows that modest alum use for pickling stays safe for most people. For those with special needs, nature’s got other crisp-makers waiting in the wings.
What are the chemical properties of Aluminium Potassium Sulfate?
Understanding Its Chemical Backbone
Aluminium potassium sulfate, often called alum, carries a formula: KAl(SO4)2·12H2O. This crystal pulls together aluminium, potassium, sulfate ions, and plenty of water locked in its structure. Each chunk bristles with water molecules, not just on the surface, but inside the lattice itself. As someone who’s worked with both raw and commercial forms, I’ve seen how this water-handling gives alum some real-world muscle.
How It Behaves in Water
Toss alum into water, and it dissolves pretty quickly. The ions break apart — you’ve got aluminium ions swirling with potassium ions and sulfate hanging around in solution. That effect stands out in old-school water purification, where alum helps clump together dirt and fine particles. From my school chemistry lab days, dumping alum into muddy water cleaned it up fast. What’s really happening? Those aluminium ions build bridges between tiny particles, dragging them out of suspension.
Alum droplets on the tongue taste sour. That comes from the acid it makes in water. The aluminium ions pull in water molecules so hard that they release protons. This creates an acidic punch — you feel it in pickling recipes and baking powders that rely on a mild but noticeable acid boost.
Reactivity and Potential Problems
Alum stays pretty chill around most chemicals. Strong bases — like lye — react fast with it, ripping apart the compound and throwing out fluffy aluminium hydroxide. I tried this trick in the lab once, and the white solid formed instantly, a clear signal that alum isn’t stable under harsh alkaline conditions. This reactivity explains why alum doesn’t get mixed into products like soaps, which tend to be alkaline.
Alum’s high solubility can cause trouble in manufacturing too. It tends to cake up in humid climates and needs airtight storage. Lose the water in those crystallized structures, and you get a powder that just doesn’t work the same. In my early days handling alum, I saw a whole bag go hard as a brick from one night left uncovered. The lesson? Pay attention to storage — moisture can undo a lot of good.
Health, Environment, and Smarter Choices
Most people run into alum in food, water filters, and cosmetics — it's GRAS (generally recognized as safe) by the FDA. Still, high concentrations can cause irritation, especially around sensitive skin or in large doses in water. Some research has looked at connections between alum and aluminium exposure for health, prompting a call for moderation rather than blanket bans.
Safe handling is the way forward. Industries must keep an eye on waste streams. Alum runoff can shift river water pH or boost metal levels that hurt aquatic life. In my own work with wastewater, small tweaks in dosing kept plant discharges in check. This offers a real fix for environmental risk: careful dosing, better tracking, and open reporting.
Looking Ahead
Alum will stick around in dozens of old and new uses. The challenge for manufacturers and communities is transparency and steady review. Better education about chemical handling and a shift to greener alternatives where possible both make sense. Lessons from the chemistry bench stick with you: even a simple salt can reshape a whole system. Respect for detail — on the shelf, in the plant, and in the environment — never goes out of style.
How should Aluminium Potassium Sulfate be stored?
Looking at This Everyday Chemical
Aluminium potassium sulfate, better known as alum, pops up in several places. Some use it for pickling at home. Others spot it in classrooms or even in water treatment plants. It isn’t as intimidating as some exotic chemicals, but it still deserves real respect. An easy mistake, like letting it mix with the wrong things or forgetting about damp conditions, can cause more headaches than people realize.
Why Proper Storage Makes a Difference
Anyone who has handled alum will tell you: it loves water. Let it sit open in a humid environment and crystals start to clump together. If you’ve grabbed a jar from your pantry that looks like a solid rock, then you’ve seen this problem. Now think about it on a shelf at a plant, where clumps could block machines or ruin a batch. Water also speeds up contamination, and that can cause trouble for whatever product the alum was meant to help purify or preserve.
On top of that, alum’s not toxic like mercury or cyanide, but it can irritate skin and eyes. The dust from fine powders lingers in the air, irritating lungs if someone isn't careful. That reminds me of an old high school science lesson where half the class sneezed during a quick experiment. In places where kids or pets might poke around, safe storage suddenly feels less like an option and more like a duty.
Simple Steps Make a World of Difference
Skip the fancy containers. A sturdy jar with a tight lid—ideally glass or high-quality plastic—keeps out dampness and keeps family or coworkers from sneaking a hand in. Nobody wants to taste homemade pickles laced with extra bugs or dirt. If you’re working someplace bigger, proper labeling stands out—a clear sticker or marker note keeps people from mixing up powders or reaching for the wrong stuff in a rush.
For those handling large amounts, a dedicated spot away from sunlight and major heat sources works best. High temperatures break down many chemicals over time and alum is no exception, especially when mixed with moisture. Designated shelves or lockers, dry indoor rooms, and sealed bags all do wonders. For home cooks, tucking the container in a cupboard above the stove sounds easy, but it’s worth fighting that habit, since heat and steam slip in fast. Store down low or out of direct light, away from the dishwasher or sink.
What the Experts and the Science Say
Reliable sources such as the Centers for Disease Control and Prevention highlight the importance of proper containment for basic household and laboratory chemicals. They regularly point out that even common substances like alum should stay out of reach from children and should never sit near food prepping stations. The U.S. Food and Drug Administration also reminds us: contamination and mishandling at any step leads to product recalls, harming reputations and wallets.
Good habits turn into routines. In my kitchen, as soon as powder goes in the jar, the lid gets snapped on. At a workplace, setting up storage protocols with a team keeps everyone on the same page. It’s a simple act that prevents unnecessary exposure and risk.
Addressing Storage Issues: Taking Real Action
Getting careless with where alum ends up can lead to dangerous cross-contamination. So schools and factories use dedicated rooms, humidity monitors, and frequent checks. Many businesses rely on regular training so that new workers handle even basic compounds with care. For folks at home, double-checking that kids can’t yank containers off a shelf offers peace of mind and prevents sudden disasters.
Clear routines, smart storage, and a little science-backed knowledge all work together. That way, aluminium potassium sulfate remains a useful tool instead of turning into a source of worry or waste.
Where can I buy Aluminium Potassium Sulfate?
Spotting the Right Supplier
Aluminium potassium sulfate doesn’t find its way onto every grocery shelf, but that doesn’t mean it’s hard to track down. Plenty of folks bump into it searching for “alum,” whether for pickling, crafts, or water purification. Grocery stores with a good canning section have small jars for pickling season. Asian markets, especially those stocking food ingredients for dessert recipes or dyeing, usually carry it near spices or preserved foods. Your best bet in person usually comes down to local hardware stores or gardening centers. Some of these stores keep larger bags for folks interested in water treatment or natural dyeing. Not every mom-and-pop shop will have it, but those with decent gardening sections often stock it for hydrangea lovers who want blue blooms.
Online, the field opens up. Websites like Amazon, eBay, and Walmart list “potassium alum” in everything from tiny spice containers to bulk industrial bags. Science supply companies and art supply shops list it under chemicals for experiments and textiles. I’ve even run into soapmaking and bath bomb hobbyists who use it for their crafts, so bath product specialty shops sometimes offer food-grade or technical-grade versions. Some chemical suppliers request a business license, so it’s worth reading each site’s terms. Just make sure to check reviews—chemical quality, shipping reliability, and price can swing wildly. If the purpose is culinary or cosmetic, food-grade quality matters.
Safety, Trust, and Expertise
Not every supplier plays by the book. Problems pop up when labeling isn’t clear or the stuff comes re-packaged with minimal information. I worry less about brand names and more about transparency—origin, purity, usage, and storage details. Food-grade alum should mention it’s fit for kitchen use. If it’s for scientific or industrial projects, the supplier ought to include a material safety data sheet. Genuine dealers will share handling advice and storage codes to keep it stable. When I’ve checked suppliers for my own projects, those who answer questions and share batch test results give me peace of mind.
Regulated retailers build reputations on safety. That’s not just about cutting out fakes—it’s about trust for the long term. Distributors with ISO certification or compliance with FDA, USP, or ACS quality standards usually say so loudly, even on their websites. Checking for customer support channels, warranty info, and detailed product listings goes a long way. I don’t just look for the cheapest bag—I want an address, a human behind the business, and communication if questions come up down the road.
Considering Practical Uses and Hazards
Folks often hunt for aluminium potassium sulfate for diverse reasons. Home picklers buy small amounts for crunchy cucumbers. Gardeners adjust soil pH with alum for their plants. Teachers use it for crystal-growing science experiments. Each use puts a slightly different burden on the supplier to offer advice. Alum isn’t risky compared to many chemicals, but swallowing a pile or mixing it with other reactants creates problems. Even common substances can backfire if instructions go unread. I stick reminders on storage containers: clear labeling and out-of-reach placement if there are kids or pets around.
Stricter online rules have cleaned up some supply chains, especially in the wake of fake consumer goods. Educational content from legitimate stores helps. More sellers include links to instructions or provide PDFs with safety and handling updates. If I see “Not for human consumption” warnings, I double check grade and intended use. Greater supplier education, clearer labeling, and a traceable supply chain always make buying any chemical easier and safer.
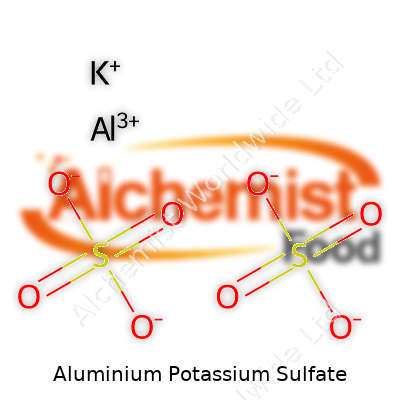
| Names | |
| Preferred IUPAC name | Potassium aluminium tris(sulfate) |
| Other names |
Alum Potassium alum Potash alum Aluminum potassium sulfate dodecahydrate |
| Pronunciation | /ˌæl.jʊˈmɪn.i.əm pəˈteɪ.si.əm ˈsʌl.feɪt/ |
| Preferred IUPAC name | potassium aluminium tris(sulfate) |
| Other names |
Potassium Alum Alum Potash Alum Aluminum Potassium Sulphate Alumen |
| Pronunciation | /ˌæl.jʊˈmɪn.i.əm pəˈtæs.i.əm ˈsʌl.feɪt/ |
| Identifiers | |
| CAS Number | 7784-24-9 |
| 3D model (JSmol) | `Al3++K+2O16S2` |
| Beilstein Reference | 1000387 |
| ChEBI | CHEBI:32599 |
| ChEMBL | CHEMBL1201147 |
| ChemSpider | 14129 |
| DrugBank | DB11110 |
| ECHA InfoCard | 100.028.856 |
| EC Number | 1332-21-4 |
| Gmelin Reference | 818 |
| KEGG | C14214 |
| MeSH | D000441 |
| PubChem CID | 24856 |
| RTECS number | BZH12030W |
| UNII | N9TZL08V8G |
| UN number | UN3077 |
| CAS Number | 10043-67-1 |
| 3D model (JSmol) | `JSmol('Al2K2O14S2')` |
| Beilstein Reference | '16911' |
| ChEBI | CHEBI:48607 |
| ChEMBL | CHEMBL1201514 |
| ChemSpider | 22675 |
| DrugBank | DB11110 |
| ECHA InfoCard | ecnumber-233-141-3 |
| EC Number | E522 |
| Gmelin Reference | 3798 |
| KEGG | C14347 |
| MeSH | D010409 |
| PubChem CID | 24856 |
| RTECS number | WS5696000 |
| UNII | F5TD010360 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DJ9LO7CO7V |
| Properties | |
| Chemical formula | KAl(SO4)2·12H2O |
| Molar mass | 474.39 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 2.67 g/cm³ |
| Solubility in water | Soluble |
| log P | '-3.72' |
| Vapor pressure | Negligible |
| Acidity (pKa) | ~3.3 |
| Basicity (pKb) | 8.2 |
| Magnetic susceptibility (χ) | −23.1·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.453 |
| Dipole moment | 0 D |
| Chemical formula | KAl(SO4)2·12H2O |
| Molar mass | 474.388 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.75 g/cm³ |
| Solubility in water | Soluble |
| log P | -4.37 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.0 (H2O) |
| Basicity (pKb) | 8.2 |
| Magnetic susceptibility (χ) | '−37.8×10⁻⁶ cm³/mol' |
| Refractive index (nD) | 1.456 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 155.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -4094 kJ·mol⁻¹ |
| Std molar entropy (S⦵298) | 155.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -4096.1 kJ/mol |
| Pharmacology | |
| ATC code | A12AB01 |
| ATC code | A12AB01 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 (oral, rat): 6207 mg/kg |
| LD50 (median dose) | 6,200 mg/kg (rat, oral) |
| NIOSH | GNDR |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2 mg/m³ |
| IDLH (Immediate danger) | Not listed |
| Main hazards | Irritating to eyes, skin, and respiratory system |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-1 |
| Lethal dose or concentration | LD50 (oral, rat): 6,200 mg/kg |
| LD50 (median dose) | 6,200 mg/kg (oral, rat) |
| NIOSH | WA1425000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2 mg/m³ |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Aluminium sulfate Potassium alum Ammonium alum Sodium alum |
| Related compounds |
Aluminium sulfate Potassium alum Ammonium alum Sodium alum Chrome alum |

