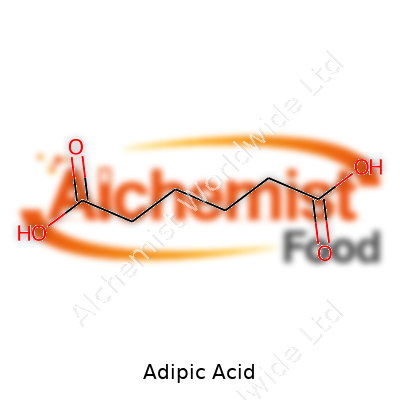
Adipic Acid: History, Production, Properties, and Its Role in Industry
Historical Development
Adipic acid has a story stretching back to the mid-nineteenth century, tracing much of its development through changing trends in chemistry and engineering. Early researchers pulled this compound from natural sources, but as demand grew, chemists began hunting for new synthesis routes. By the 1940s, large-scale petrochemical processing replaced earlier, less predictable fermentation methods. This change didn’t just fuel nylon’s invention—it rapidly expanded the market for synthetic polymers. The explosive growth in post-war consumer goods put adipic acid squarely on the map as a central ingredient in everything from clothing fibers to engineering plastics.
Product Overview
Adipic acid falls into the dicarboxylic acid category and stands out as a staple in modern materials manufacturing. Its six-carbon chain gives it useful flexibility compared to shorter diacids, which shows up in everything from softer nylon to food additives and lubricants. Most folks encounter it every time they pull on a nylon-based textile, but it hides in chewing gum, gelatins, some pharmaceutical formulations, and even specialty coatings. Chemists value it because the molecule opens doors to a wide mix of polymers and polyurethanes, and engineers rely on its predictable melting and solubility behaviors in scaled-up production.
Physical & Chemical Properties
This white, crystalline powder carries a faintly tart flavor, sometimes used as an acidulant in foods. Its melting temperature falls just below 153°C, which makes it easy to shape or transport without elaborate cooling systems. Adipic acid barely dissolves in cold water, but it mixes more freely with hot water and polar solvents like acetone and methanol. In lab settings, this trait lets chemists separate or purify it without exotic procedures. Its molecular weight sits at 146.14 g/mol, and in my experience with practical handling, static and clumping rarely interfere with typical benchwork or industrial storage. Its pKa values rest at about 4.41 and 5.41, a detail that lets chemists fine-tune reactions and downstream modifications with confidence.
Technical Specifications & Labeling
Companies label bags and barrels of adipic acid by purity, particle size, and color index, not just simple chemical identity. Industrial-grade batches usually clock in above 99.7% purity, with low color values to prevent yellowing in sensitive end products. Careful monitoring of moisture and trace metals helps producers maintain solid quality year after year. Global standards like REACH in Europe, China’s GB/T norms, and U.S. FDA guidelines lock in quality, traceability, and safe handling from factory floor to downstream conversion. Standard placards spell out hazard classes (mild irritant, not acutely toxic), storage advice, and disposal rules, helping logistics teams move huge quantities safely around the globe.
Preparation Method
Nearly all commercial adipic acid now comes from a method centered on cyclohexane oxidation. Production lines start with cyclohexane, transforming it into a blend of cyclohexanol and cyclohexanone under controlled oxygen and pressure. Later, air or nitric acid finishes the process, carving these precursors down to raw adipic acid and releasing nitrous oxide as a side effect. Environmental concerns push some big names in the field to try newer methods: bio-based feedstocks (like glucose fermentation with engineered yeasts), membrane electrolysis, or catalytic oxidation without nitrous oxide emissions. These new methods compete against decades of process know-how, big up-front investments, and existing plant infrastructure. But the pressure to curb greenhouse gases and shift to renewable lines puts genuine momentum behind these alternatives.
Chemical Reactions & Modifications
Adipic acid acts as a building block for a wide range of chemical transformations. With its two carboxyl groups, it easily forms esters, amides, salts, and even more complex chains for research purposes. In my experience as a lab researcher, one of the most common transformations involves reacting adipic acid with hexamethylene diamine, yielding nylon 6,6—a workhorse material for textiles, films, and automotive parts. But the molecule’s flexibility lets it morph into polyurethanes, lightweight flexible foams, tough elastomers, adhesives, and even some hydraulic fluids. Adipic acid’s chemical behavior also includes some less glamorous sides, like partial decarboxylation under strong heating, which sometimes leads to unwanted byproducts if process controls slip.
Synonyms & Product Names
Adipic acid appears under many product names and labels. In regulatory language, you’ll see terms like hexanedioic acid, but in factories and on shipping manifests, it runs by shorter titles: 1,6-hexanedioic acid, DA, or even just “nylon acid.” Chemists sometimes abbreviate it as AA, especially when discussing bulk polymer production or comparing to similar diacids like sebacic or succinic. This collection of names sometimes confuses new lab techs, but after years of paperwork and problem-solving, those names all point back to the same essential compound.
Safety & Operational Standards
Direct contact with adipic acid has mild effects: some irritation to skin and eyes, slight reaction from airborne dust. Most industrial plants rely on local exhaust ventilation, gloves, and dust masks, which keep incident rates low. I’ve seen older plants struggle with dust suppression, especially in dry or windy climates, leading to cleanup headaches and minor medical reports. Regulatory agencies like OSHA in the U.S., ECHA in Europe, and local equivalents elsewhere mandate exposure controls and safety data sheet (SDS) labeling. Spills usually sweep up dry or dissolve in water, and the compound presents minimal risk for groundwater. Burning or illegal dumping create bigger risks—creating acrid smoke and air pollutants, so fire teams and waste handlers in the field take strict precautions.
Application Area
The lion’s share of adipic acid lands in nylon 6,6 production—feeding textile fibers, carpeting, tire cords, and injection-molded engineering plastics. If I look around my home, I spot its touch in toothbrush bristles, luggage zippers, and car door fabrics. Some smaller but persistent uses turn up in polyurethane resins, flexible foams for furniture and bedding, even as a gelling agent for pharmaceuticals. Food scientists sometimes turn to it as an acidulant or flavor enhancer, where regulations cap total intake to minimize health questions. Catalysis experts and researchers love its fine control over reactivity, using it as a backbone to build more exotic esters and polymers for high-performance films and automotive coatings.
Research & Development
Ongoing research into adipic acid focuses on cleaner, greener synthesis methods and new end uses, reflecting concerns about sustainability and climate change. I’ve spoken with university labs and startup teams working on fermenting sugars or even captured CO2 into adipic acid, aiming to bypass petroleum entirely. These newer approaches still need to match classic chemical yields and economic scale, though pilot plants continue to crop up each year. In materials science circles, new copolymers and smarter blending strategies keep showing up in journals, promising lighter, tougher, or more recyclable products for the circular economy. Most of the action remains in process intensification—shrinking energy costs, minimizing emissions, and searching for catalysts that handle less-than-perfect feedstocks with fewer headaches.
Toxicity Research
Adipic acid sits in the lower range of acute toxicity. Past studies in rodents and primates found mild effects at very high doses, mainly gut irritation or slight metabolic shifts. Regulatory bodies in North America, Europe, and Asia rank it as “generally recognized as safe,” with large safety margins for food and consumer items. Of course, dust from large spills or process failures can irritate workers or local residents, but I’ve never encountered mass poisoning or cancer links from legitimate use. Ongoing animal studies and rare workplace incidents always draw careful scrutiny from toxicologists, and the data so far keeps risks low as long as safety guidelines stay tight. Environmental groups pay closer attention to possible impacts from nitrous oxide emissions in older process plants than to direct chemical exposure.
Future Prospects
Rapid growth in sustainable materials and carbon-neutral chemistry will shape adipic acid’s destiny for decades ahead. Petrochemical-based supply chains face mounting regulatory and reputational pressure, especially in markets where carbon footprints drive buying decisions. The field will not shift overnight, but every company with a big stake in high-performance polymers keeps a watchful eye on fermentation-based and waste-free synthesis routes. Consumer trends in textiles, 3D printing, and electric vehicle parts all raise demand for lighter and more resilient polyamides, locking adipic acid in as a building block with permanent relevance. As policymakers push for stronger recycling infrastructure and “green” labeling, the technology race to make cleaner, smarter adipic acid won’t let up anytime soon.
What is adipic acid used for?
Building Block of Everyday Life
Many folks might walk right past a chemical like adipic acid without much thought. I used to do the same until I started reading the fine print on household goods. Adipic acid shapes plenty of what we touch and use every day. If you ride in a car or step into a kitchen, there’s a high chance you’re experiencing the results of this powdery compound. Somewhere along the way, you sit on a seat made from nylon or store leftovers in a plastic container shielded by the science that started with this acid.
Sneakers, Tires, and Zippers: Nylon Relies on Adipic Acid
The biggest use sits smack in the world of nylon. Engineers mix adipic acid with hexamethylenediamine and, through a bit of heat and patience, nylon appears. Nylon ropes carry heavy loads, tents stand up to pelting rain, and car airbags save lives thanks to the fabric’s tough roots in basic chemistry. I once had a jacket that outlasted three winters, all because the zipper could take more punishment than the coat itself. That strength starts with adipic acid’s role in producing nylon 6,6—a tough, resilient plastic fiber found everywhere from parachutes to guitar strings.
Quietly Shaping Food and Pharma
Adipic acid sneaks into food too. Ever noticed a tart kick in gelatin desserts or powdered drinks? That zing often owes itself to this same chemical. It’s valued for turning limp, bland mixtures into something a little brighter. Companies use it because it keeps flavors stable and consistent in ways lemon juice or citric acid can’t always match. In my days helping to coordinate food safety workshops, I saw bakers and food technologists trust this ingredient to keep their mixes just right.
Pharmaceutical companies sometimes turn to adipic acid as a stabilizer. A steady release of medicine makes a big difference for anyone managing chronic issues. By including this acid, they can control how fast and how much medicine releases—something every patient wants when dealing with time-sensitive drugs.
The Environmental Cost
No honest talk about chemistry dodges the bigger questions. Policymakers and scientists face tough calls with adipic acid. Every year, industries pump out over three million tons and, during its production, release nitrous oxide—a greenhouse gas strong enough to draw headlines. The United Nations recognizes nitrous oxide as a significant factor in climate change. The impact isn’t lost on anyone who watches their local river or tracks urban air quality reports. The same chemical that helps keep a car’s upholstery in place also challenges our ability to keep the air clean.
New Approaches on the Horizon
Solutions do exist. Some manufacturers try to cut emissions by capturing the nitrous oxide and breaking it down before it leaves the smokestack. Others turn their sights toward biobased processes, using plant sugars to make adipic acid instead of traditional oil sources. I’ve sat in on panel discussions where young scientists argue the merits of fermentation, and it’s hard not to feel a bit of hope. Cleaner production methods don’t just mean shaking up chemistry labs—they touch jobs, health, and how much trust we put in innovation.
Paying Attention Pays Off
Adipic acid may sound technical, but it shapes things we all depend on. Whether you’re lacing up running shoes or planning a family meal, this compound has likely played a part. Understanding its uses, risks, and path forward lets people make smarter choices—on the store shelf and the ballot.
Is adipic acid safe for human consumption?
What Is Adipic Acid Doing in Food?
Most folks aren't checking labels for adipic acid, but this compound pops up in surprising places: powdered drinks, processed cheeses, even bakery mixes. As someone who’s spent time in food labs and supermarket aisles alike, I’ve seen how manufacturers use food acids like this to adjust flavors and keep products shelf-stable. Adipic acid, a white crystalline powder, delivers a mild sourness and helps get the right texture in jellies or drink powders. Its low bitterness means kids and adults don’t notice it, which helps products appeal to a wider range of tastes.
The Science Behind Adipic Acid
Every time people ask, “Is this safe?” they're really wondering how the body handles it. Scientists looked long and hard at adipic acid, since it's a synthetic additive. Reputable agencies, including the U.S. Food and Drug Administration and the European Food Safety Authority, investigated the effects on living systems. They set a daily intake threshold as a safety net. The numbers stack up favorably: according to current studies, you’d need to eat more processed food than most people ever would just to approach the recommended upper intake.
The body handles small amounts of adipic acid by breaking it down in the liver. It leaves the system through regular metabolic pathways, with no evidence for buildup—unlike some other chemicals we're told to watch out for. Its history stretches back decades, making it a known quantity in both food technology and toxicology research.
Risks—Real or Hyped?
It’s easy to lump adipic acid with industrial-sounding ingredients and raise eyebrows, but context matters. At normal levels in food, the overwhelming evidence says it's not hazardous. The real trick is quantity. Animal studies show extremely high doses—far beyond what anyone could get from food—can cause minor digestive upset, but not systemic harm. People who already deal with sensitive stomachs sometimes report mild irritation after eating foods high in acid content in general, but that comes from the cumulative effect of multiple ingredients.
There was an isolated study floating around a few years ago that suggested breakdown products could, in theory, contribute to nitrosamine formation if mixed with certain preservatives under high-heat conditions. Real-world scenarios don't match these lab setups. Still, concern about mixing certain additives gave regulators the push to require clear labeling and encourage more research on new uses.
Solutions for Cautious Consumers
Food shoppers who want to steer clear of additives can stick to whole foods without extra acids or preservatives. Cooking at home with fresh, simple ingredients keeps your exposure to a minimum. Parents worried about picky eaters getting too much from flavored drinks can check labels—adipic acid must be listed if it’s present in the product.
Pushing for better studies always helps. Demand for transparency drives responsible use of food additives. Companies with their eye on consumer trust now publish more about sourcing and quality checks than ever before, allowing interested eaters to trace ingredients back to origin and processing steps. Advocacy for clearer nutrition information gets stronger every year, making it easier to make informed choices without a chemistry degree.
How is adipic acid manufactured?
A Chemistry-Driven Cornerstone of Everyday Life
Adipic acid plays a key part in producing nylon, certain food additives, and makes its way into everything from car tires to artificial flavorings. People cross paths with it more often than they realize, although they probably never stop to think about what’s involved in making it. The most common path from raw material to product uses one of the classic workhorses of industrial chemistry: nitric acid oxidation of cyclohexanol and cyclohexanone. The process isn’t pretty, and as someone who’s spent time around chemical manufacturing, it’s impossible to escape the realities behind its environmental impact.
The Chemistry Under the Microscope
At the plant, adipic acid production starts with cyclohexane as a feedstock, often sourced from petroleum. This cyclohexane undergoes oxidation, generally yielding a mixture of cyclohexanol and cyclohexanone, sometimes just called “KA oil.” Next, the chemistry gets more serious with nitric acid. The KA oil reacts with nitric acid, generating adipic acid as the star product. This route leads to a pretty substantial byproduct—nitrous oxide, a greenhouse gas packing nearly 300 times the warming effect of carbon dioxide.
I’ve seen firsthand how industrial plants need to manage these emissions. Nitrous oxide gets vented into the atmosphere if not scrubbed or abated. Technology makes it possible to recover and decompose some of it, but older facilities often lag behind, lagging under the weight of legacy processes and outdated equipment. It drives home how chemical manufacturing, as much as it makes daily comforts possible, brings along challenges that simply can’t be wished away.
Hard Choices and Real Solutions
Clearing up this environmental footprint takes more than talk. Modern engineering improvements exist. Some plants use catalytic decomposition to break down nitrous oxide before it escapes into the air. It’s a fix that shows up mostly at newer facilities or in places with strong regulations. The hard reality is that retrofitting older plants costs serious money, and corners sometimes get cut, especially when profit margins get squeezed.
There’s energy use to consider too. Heating, cooling, and moving around large volumes of acid, water, and solvents guzzle a lot of power. Chemical manufacturing always feels like a balancing act—run the process clean, and the bills shoot up; focus on costs, and pollution creeps in. In the lab, alternative routes keep popping up. Biotech has offered ideas: fermentation using engineered microbes that convert glucose directly to adipic acid. These ideas sound promising, and a few pilot plants have cropped up. Still, scaling this up to the level of the old-school nitric acid method hasn’t fully worked out yet, mostly due to cost or yield snags.
The Road Ahead
Fact is, the story of adipic acid mirrors the big dilemma of modern industry. Demand never lets up, but the urgency to run cleaner is growing by the year. Governments and the public are watching closer than ever. A few forward-thinking companies have started to partner with universities and independent scientists to optimize process flows and reduce climate impacts. My experience tells me that meaningful change comes from both outside pressure and the pride the best engineers put into their work. Every step toward lower emissions, whether it’s through smarter chemistry or stricter controls, matters more now than it did a decade ago.
It’s easy to see this as just another science problem, but there’s more at stake: the choices made in plants and labs ripple outward, affecting the air people breathe and the world their kids inherit. The reality of adipic acid manufacturing isn’t glamorous, but the stakes are real—and so are the opportunities to lighten its footprint.
What industries commonly use adipic acid?
The Backbone of Modern Manufacturing
Adipic acid might not grab headlines, but it’s a workhorse in several industries. Every time I lace up my sneakers or plug in an appliance, I’m reminded how this compound keeps daily life running smoothly. Factories crank out more than two million tons of adipic acid each year. That kind of volume points to its broad demand and impact on the products we use every single day.
Textiles: From Polyamide Fibers to Durable Clothes
Most people don’t look at a jacket or carpet and wonder about the chemicals in the fibers. But I’ve seen how crucial adipic acid is while working with textile engineers. Nylon, especially Nylon 6,6, owes its toughness and flexibility to adipic acid. Factories mix it with hexamethylenediamine, form the polyamide, and then spin strong, lightweight fibers that make up activewear, ropes, and car airbags. The reliability of these fibers supports everything from safer driving to intense gym sessions.
Plastics and Automotive: Beyond Flexible Hoses
Walk through any car assembly line and you’ll find components shaped by adipic acid. Car parts like seat coverings, under-the-hood connectors, and flexible hoses require polymer resins for strength and endurance. In my own garage, I’ve fixed broken containers and cables—made from polyurethanes or engineering plastics—where adipic acid brings flexibility and resistance to wear. Joints and rollers in furniture, wheels in skateboards, and packaging films also depend on it.
Food and Beverages: More Than Just an Additive
Some food products lean on adipic acid as a flavor enhancer and gelling aid. If you’ve tried sugar-free gelatin, you’ve tasted the tang adipic acid brings to the table. Foods with a longer shelf life—like jellies and powdered drinks—often include it to control pH and create the right texture. I’ve looked through ingredient lists for years as a parent shopping for lunchbox snacks, and I spot this name more often than most would guess.
Pharmaceuticals: Helping Tablets Go Down Smoothly
Pharmaceutical labs benefit from adipic acid in ways you might not expect. While consulting on tablet production lines, I noticed that manufacturers use it to improve tablet compressibility and taste. Additives like these ensure medications remain palatable and stable from the factory to your medicine cabinet.
Cleaning Products: Powering Detergents and Bleaches
Adipic acid acts as a bleaching agent in detergents. After chatting with chemical engineers and touring household cleaner plants, I saw how it helped soften water and enhance stain removal. As a result, disinfectants rely on it for gentler action on clothes and surfaces. That shelf of cleaning supplies at home? They owe some of their effectiveness to this essential compound.
Concrete and Construction: Supporting Infrastructure
Adipic acid derivatives show up at construction sites. Concrete admixtures and plasticizers help builders pour strong, workable concrete. Speaking with civil engineers, I learned how this improvement reduces cracks and boosts durability—crucial for everything from bridges to foundations.
Moving Toward Sustainable Use
The ongoing challenge lies in balancing utility with environmental stewardship. Traditional production methods give off significant nitrous oxide, a greenhouse gas. Chemical companies now invest in greener manufacturing—such as biobased processes—helping reduce the environmental impact while keeping industries rolling. More companies and consumers keep asking the tough questions about ingredient sourcing, safety, and sustainability, pushing for innovation that benefits both people and the planet.
What are the storage and handling recommendations for adipic acid?
Understanding the Stuff: Adipic Acid Isn’t Harmless
Adipic acid plays a big role in making nylon, plasticizers, and food additives. It comes as a white, powdery solid that feels pretty basic to handle, just like table sugar at first glance. But don’t be fooled—the safety sheet tells another story. Breathing in the dust can bother the lungs and eyes. Some workers have dealt with coughs or itching after exposure. That means there’s sense in taking its storage and handling seriously. Accidents cost people health, money, and time, so every manufacturer or warehouse should put real thought into safety routines.
No Room for Moisture or Heat
Moisture is the enemy for chemical powders like this one. Adipic acid absorbs water and clumps up over time, making it hard to measure and mix. If left in a humid room or storeroom, it can start to cake or even partly dissolve. Keeping the container tightly sealed and stored in a dry spot makes a difference. Workers often use dehumidifiers or silica desiccants in humid places. Removing excess moisture in a storage room helps keep product quality stable and ensures batches mix evenly.
Heat packs another punch. Adipic acid melts around 152°C, but it starts breaking down at much lower temperatures. Factory workers learned the hard way that leaving it near boilers, radiators, or gear that heats up can make it clump or even release fumes over time. A key habit is putting bags or drums away from any heat sources. Even sunlight sneaking through a window risks raising the temperature too much, so shaded indoor storage is a common-sense step.
Container Choices and Labeling
Paper bags with polyethylene liners, plastic drums, or even fiber drums offer solid protection—as long as they seal tightly. Every time I’ve worked around chemicals, double-checking the seal has saved more headaches than I can count. Once moisture slips in, entire batches go to waste.
Clearly labeling every container and drum matters. A simple tag showing what’s inside, the hazard class (it’s not highly flammable, but it’s mildly acidic), and the date received keeps everyone on the same page. This isn’t about bureaucracy; it’s about stopping costly mix-ups—no one wants to see adipic acid end up where it shouldn’t go.
Good Habits in the Work Zone
Dust matters most during transfer—pouring from sacks, loading hoppers, and dealing with spills. Proper ventilation, dust masks, and avoiding open pours limit exposure. I’ve seen workers spray down floors to settle dust before sweeping—an old-school trick, but it works. Training new staff on these routines prevents long-term health issues and keeps insurance claims down.
Handwashing before lunch or leaving the area is a tiny step with major payback. Folks bringing chemical dust to the break room or parking lot open up risk. Simple lockers and handwashing stations close to the work zone really help.
A Culture Shift: Less Cheap, More Smart
Cutting corners on storage might look like a money-saver in budgets, but spoiled lots and worker injuries end up costing far more. Factory managers who push for better airflow, better sacks, and better labeling see returns on lower waste and fewer complaints. The real solution lies in building habits that treat chemicals with the respect they deserve: no shortcut, no gamble, and no guesswork. That includes safer containers, training, and a few common-sense checks at the start and end of every shift.
People often forget the simplest routines make the best defense. Double-check a seal, wipe a bench, label what you move—that's where safe chemistry starts.
| Names | |
| Preferred IUPAC name | hexanedioic acid |
| Other names |
1,6-Hexanedioic acid Hexanedioic acid Acid of Oil of Vitriol Adylic Acid Tetrahydrofumaric Acid |
| Pronunciation | /ˈæd.ɪ.pɪk ˈæs.ɪd/ |
| Preferred IUPAC name | hexanedioic acid |
| Other names |
Hexanedioic acid 1,4-Butanedicarboxylic acid |
| Pronunciation | /ˈæd.ɪ.pɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 000124-04-9 |
| Beilstein Reference | 695786 |
| ChEBI | CHEBI:30794 |
| ChEMBL | CHEMBL472 |
| ChemSpider | 5285 |
| DrugBank | DB01862 |
| ECHA InfoCard | 03e90baf-9e76-4f7d-80cc-bd9b8e8588ff |
| EC Number | 204-673-3 |
| Gmelin Reference | 69226 |
| KEGG | C01547 |
| MeSH | D000255 |
| PubChem CID | 196 |
| RTECS number | AR9100000 |
| UNII | V57H60VW2B |
| UN number | UN2076 |
| CAS Number | 124-04-9 |
| Beilstein Reference | 1720802 |
| ChEBI | CHEBI:30772 |
| ChEMBL | CHEMBL1407 |
| ChemSpider | 643 |
| DrugBank | DB01877 |
| ECHA InfoCard | 100.003.467 |
| EC Number | 204-673-3 |
| Gmelin Reference | 79398 |
| KEGG | C01547 |
| MeSH | D000246 |
| PubChem CID | 196 |
| RTECS number | AR9100000 |
| UNII | V57H60P9DW |
| UN number | UN2076 |
| Properties | |
| Chemical formula | C6H10O4 |
| Molar mass | 146.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.36 g/cm³ |
| Solubility in water | 14 g/L (20 °C) |
| log P | -0.29 |
| Vapor pressure | 0.001 mmHg (20°C) |
| Acidity (pKa) | 4.41, 5.41 |
| Basicity (pKb) | 2.91 |
| Magnetic susceptibility (χ) | -7.4×10⁻⁶ |
| Refractive index (nD) | 1.439 |
| Viscosity | Viscous solid |
| Dipole moment | 1.12 D |
| Chemical formula | C6H10O4 |
| Molar mass | 146.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.36 g/cm³ |
| Solubility in water | 14 g/L (20 °C) |
| log P | -0.29 |
| Vapor pressure | 0.13 mmHg (at 25 °C) |
| Acidity (pKa) | 4.41, 5.41 |
| Basicity (pKb) | 1.09 |
| Magnetic susceptibility (χ) | -7.9·10⁻⁶ |
| Refractive index (nD) | 1.439 |
| Dipole moment | 1.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 157.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1277.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3351.8 kJ/mol |
| Std molar entropy (S⦵298) | S⦵298 = 248.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1276.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3351 kJ/mol |
| Pharmacology | |
| ATC code | A16AX10 |
| ATC code | A16AX10 |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P210, P280, P264, P301+P312, P305+P351+P338, P330, P501 |
| Flash point | 196°C |
| Autoignition temperature | 400 °C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Oral Rat 5560 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5700 mg/kg (oral, rat) |
| NIOSH | AY3675000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Adipic Acid: 5 mg/m³ |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | IDLH: 5,000 mg/m³ |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 196°C |
| Autoignition temperature | 410 °C (770 °F) |
| Lethal dose or concentration | LD50 Oral Rat 5700 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 5700 mg/kg |
| NIOSH | NA0825 |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 0.5 mg/m³ |
| IDLH (Immediate danger) | 500 mg/m3 |
| Related compounds | |
| Related compounds |
Sebacic acid Glutaric acid Succinic acid Terephthalic acid Phthalic acid |

