Acetohydroxamic Acid: A Journey Through Science and Application
Historical Development
Acetohydroxamic acid, often referred to as AHA, began turning heads in the early twentieth century as chemists started poking around for niche substances that could break tough chemical bonds. Its deliberate synthesis came during research into ligands and complex formation chemistry, areas that gained traction with the rapid development of nuclear technology after World War II. At the time, scientists scrambling to control uranium separation found acetohydroxamic acid to be especially handy due to its ferocious appetite for iron and other metals. Universities and national labs collected patents, and research studies flooded in on possible uses, setting the stage for its medical debut a few decades later.
Product Overview
Acetohydroxamic acid stands out as a small molecule — technically a hydroxamic acid derivative — and shows up as a white crystalline powder. It’s sold under several trade names and generic pharmaceuticals. Its breakthrough came with the approval for use in humans, specifically to deal with infection-driven urinary stones, showing that small discoveries in obscure corners of chemistry can drive solutions where others failed. Key pharmaceutical companies manufacture it under well-controlled conditions, usually shipping as tightly sealed vials or drums to prevent moisture uptake and degradation.
Physical & Chemical Properties
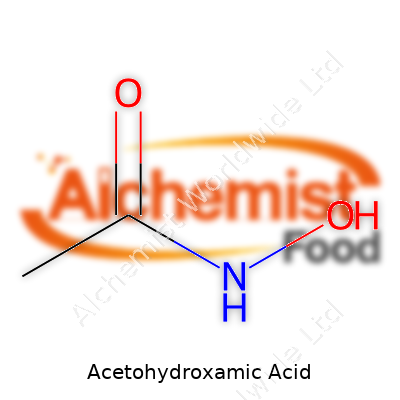
Chemically, acetohydroxamic acid has the formula CH3CONHOH. It melts just above room temperature, around 58–62°C, which means improper storage quickly turns it into a sticky mess. It dissolves easily in water thanks to its polar structure — a vital trait since most applications, especially medical ones, require dissolving it completely. The crystalline appearance throws off an almost bitter-almond scent, noticeable when opened in a typical lab. Strong chelating power lets this acid bind tightly to a variety of metals. Anyone handling it in bulk quickly appreciates its hygroscopic nature; just a few minutes exposed air leaves clumps and caking, which points to the reason why strict storage matters.
Technical Specifications & Labeling
Products hit the lab bench with purity ratings hovering around 98–99%, because minor impurities can trigger unwanted reactions or trip up regulatory approval. Pharmaceutical-grade AHA comes screened for heavy metals and residual solvents, and the labeling reflects not only lot numbers but also origin, date of synthesis, and recommended storage temperature. Regulatory bodies in the United States and China, for instance, insist on detailed safety sheets—sections that outline the proper personal protective equipment and waste handling based on region-specific requirements.
Preparation Method
Most labs and factories synthesize acetohydroxamic acid via the reaction of ethyl acetate with hydroxylamine under acidic or neutral conditions. Some use acetic anhydride instead, claiming greater yields or fewer byproducts. This works well at moderate temperatures, letting industry crimp down on side-products like acetamide. After the initial reaction, acetic acid leftovers require neutralization, and then purification steps — usually recrystallization from ethanol or water. Drying under vacuum seals the deal. Research chemists constantly debate subtleties in the reaction path, pointing to the way small changes in pH or temperature lead to higher purities and more stable products.
Chemical Reactions & Modifications
AHA’s value shines through in its chemistry — the hydroxamic group (–CONHOH) latches on to metal ions, which disrupts many metal-catalyzed biological and industrial reactions. This chelation capacity grants it the ability to knock out unwanted side-products, or block specific pathways in biological systems. Chemists sometimes swap the acetyl group for larger alkyl or aryl pieces, creating analogues that target different metals or boost stability. Over decades, modifications to the backbone have led to derivatives with unique medicinal properties, and patent literature contains dozens describing tweaks for better selectivity or lower toxicity.
Synonyms & Product Names
Among chemistry circles, acetohydroxamic acid crops up under names like N-hydroxyacetamide, AHA, or even simply acetic acid hydroxamate. Pharmacies label formulations by brand names in clinical settings. Regulatory registries in Europe and Asia assign it with codes and standardized identifiers, keeping things consistent between chemical catalogs, trade regulations, and clinical trial reports. Synonyms exist partly to sidestep confusion between closely related hydroxamic acids, which sometimes get mistaken for each other due to similar molecular arrangements.
Safety & Operational Standards
Every handler must take safety seriously. Lab accidents often stem from underestimating skin or eye contact risk; AHA causes irritation and triggers rashes, so eye protection and gloves must go on, even for brief work. Exposure to airborne dust can cause breathing problems, making fume hoods mandatory in production or research settings. Its solid form can combust under the wrong conditions, and researchers often report cases where improper disposal contaminated workspaces. Storage in tightly capped containers, away from sunlight and humid air, heads off most dangers. Regulatory agencies require routine training, auditing safety processes, and updating hazard communications every few years.
Application Area
Medicine takes top billing for acetohydroxamic acid, as the compound acts as a powerful inhibitor of the enzyme urease — a key player in urinary tract infections and subsequent stone formation. Patients with persistent infection or catheters rely on AHA to keep waste products from crystallizing. Beyond medicine, its strong chelation properties find a role in separating metals during nuclear fuel reprocessing, especially when scientists need to separate uranium and plutonium. Environmental chemists test it as a treatment for heavy metal poisoning in industrial spills. Fiction writers rarely hint at such a broad reach for compounds most see as “just another white powder,” yet this acid moves seamlessly from bio-medical uses to reactor-grade chemistry.
Research & Development
Academic groups across continents search for new roles for acetohydroxamic acid. Some focus on tweaking its structure, betting that a slight shift in functional groups will unlock fresh bioactivity or reduce unwanted side effects in patients. Others eye environmental chemistry, where AHA’s metal-binding impresses as a possible solution for wastewater cleanup after battery manufacturing or mining. At scientific conferences, heated debate rages about how much toxicity reduction these modifications actually achieve—every improvement lands under peer review, forcing refinements and keeping long-term standards high. Clinical work investigates new delivery systems, such as slow-release granules or implant coatings, to stretch its clinical window and bring relief to stubborn patient populations.
Toxicity Research
Doctors and toxicologists watch acetohydroxamic acid’s use like hawks, never trusting that a useful drug will stay safe if used carelessly. Reports document cases of fever, headaches, rash, and rarely, bone marrow suppression or blood disorders after long-term use. Even in laboratories, chronic exposure leads to complaints of fatigue or allergy-like symptoms. Dosing limits reflect these findings, with countries allowing only short courses or asking for extra monthly blood monitoring. Animal studies confirm the organ-specific toxicity, most notably in the liver and kidneys, which feeds into debates over whether further structural modification might blunt some of these effects. Safety testing, postmarketing surveillance, and constant reevaluation help keep risks in check, but the stories from real patients and workers never fade from regulatory memory.
Future Prospects
Looking past today’s boundaries, opportunities knot together in both medicine and industry. Newer antibiotic-resistant bacteria drive the hunt for stronger, safer urease inhibitors, and AHA-inspired molecules land at the front of that line. In the laboratory, bioengineers tinker with formulations to build more targeted delivery, making complications less frequent or severe. Green chemists see a future where modified acetohydroxamic acids wrest toxic metals out of contaminated soils or drinking water, leveraging its natural affinity with iron, copper, and manganese. As interest swells around sustainable nuclear energy, demand for nuanced metal separation techniques keeps this family of chemicals relevant in fuel recycling. Success depends not just on elegant chemistry or clever marketing, but on a willingness to test new boundaries, collect mountains of data, and keep an open channel between manufacturers, clinicians, and environmentalists. Acetohydroxamic acid’s story continues as a live experiment—one unrolling across clean rooms, hospitals, and remediation sites the world over.
What is Acetohydroxamic Acid used for?
Looking at Why It Matters in Medicine
Most people have never heard of acetohydroxamic acid until a doctor mentions it. This little-known medicine serves a clear, focused purpose: it helps people who suffer from chronic urinary tract infections caused by kidney stones, especially so-called “struvite stones.” These stones form mostly in folks with repeated infections and stubborn bacteria in their urinary tract.
I remember a patient in a urology clinic—a woman who had been battling recurrent infections for years, all thanks to stones that kept returning. Standard treatments—antibiotics, procedures—just wouldn’t let her catch a break. For her and people in similar shoes, using acetohydroxamic acid gives another shot at breaking the vicious cycle.
How Acetohydroxamic Acid Works
This medicine does something rather unique. Instead of fighting the infection head-on, it steps upstream and stops certain bacteria from causing trouble. Bacteria that form struvite stones use an enzyme called urease to split urea into ammonia. The ammonia boosts the pH balance in urine, making a perfect spot for stones to grow. Acetohydroxamic acid puts the brakes on that enzyme, which slows the growth of new stones. Without this medicine, patients can find themselves trapped in a cycle of infection and hospital trips.
Statistics back up its impact: Struvite stones show up in about 10% to 15% of kidney stone sufferers. Many of these folks deal with stones again and again, struggling with pain and risky infections. Antibiotics kill bacteria, but don’t always keep the stones from forming. In hard cases, acetohydroxamic acid becomes the lifeline.
Points to Watch For
Just like any strong tool, acetohydroxamic acid brings side effects. Doctors ask patients to watch for issues like headaches, stomach upset, or trouble with focus. In rare cases, it can affect the blood and kidneys if used long-term, so doctors check lab results regularly to catch early signs of trouble. I’d want anyone taking it to understand their treatment plan and never hesitate to ask questions or share new symptoms.
Addressing the Root Problem
Treating struvite stones with this medicine doesn’t erase the need for infection control and stone removal. Urologists usually combine two or more approaches: surgery to take out stones, long-term antibiotics to keep bacteria at bay, and dietary changes to manage risk factors. The Cleveland Clinic and other respected centers recommend using acetohydroxamic acid in tough cases—a sign that it’s not a go-to medicine for everyone, but it’s crucial for patients whose stones resist standard care.
Don’t Go It Alone
Patients often feel overwhelmed facing unusual or chronic diseases. Having seen the difference that clear information makes, I urge anyone offered this treatment to talk it out with their doctors. Family doctors, urologists, and pharmacists can all help sort out risks, benefits, and other medicines that might create side effects.
Medical breakthroughs sometimes hide in unassuming names. Acetohydroxamic acid won’t show up in TV commercials, but it has made a quiet, steady difference for people living with strained kidneys from stubborn stones. Patients owe it to themselves to learn about every part of their treatment—especially the unsung tools that buy them more comfort, fewer emergencies, and better days ahead.
How does Acetohydroxamic Acid work?
A Real-World Look at a Small but Potent Molecule
Acetohydroxamic acid often slips under the radar in medical discussions, but for people dealing with specific urinary tract issues, it's a heavy hitter. Sometimes life brings chronic infections, kidney stones, or tricky bacteria that stick around no matter what antibiotics are thrown at them. Years ago, I had a close friend who battled repeated urinary tract infections due to long-term catheter use. Standard drugs offered no lasting relief. That’s when her doctor considered acetohydroxamic acid. Her story stuck with me, because this wasn’t just medicine from a bottle—it was a lifeline for quality of life.
The Science Behind the Relief
Acetohydroxamic acid doesn’t act like the typical bacteria-zapper most people imagine. Its specialty lies in outsmarting certain bacteria, not just killing them outright. Some bacteria, especially ones that settle in the urinary tract, thrive by producing an enzyme called urease. This enzyme turns urea into ammonia, and that ammonia creates a friendly environment for stone formation and persistent infections.
Acetohydroxamic acid steps in by blocking urease. No urease, far less ammonia. The environment in the urinary tract shifts, making life harder for troublesome bugs and reducing the risk of new stones forming. Infections lose their secret weapon. That’s why, for patients dealing with so-called “struvite stones” or infected kidney stones, this medicine can make a world of difference.
Why People Need Options Like This
Dealing with chronic urinary infections can grind a person down. Constant doctor visits, endless rounds of antibiotics, and the looming threat of kidney stones can eat away at anyone’s patience. Acetohydroxamic acid isn’t for everyone. People with liver issues or certain blood disorders need to stay away. Some folks run into side effects like headaches, stomach pain, or rashes. These stories matter, because risks aren’t just numbers—they’re real problems that might send someone back to square one.
At the same time, options matter. Hospital stays due to infected stones cost patients time, money, and energy. One recent study in the Journal of Urology pointed out that blocking urease this way reduced recurring stone formation and infection in high-risk groups over several months, lessening complications and, in some cases, surgery risks.
Room to Improve
People still struggle to get information about medicines like this. Sometimes doctors don’t mention it until all else fails, leaving patients feeling like guinea pigs. A bigger push for patient education could help. Reliable, up-to-date facts help people weigh their options and decide whether the possible discomforts of the drug beat out the threat of long-term infection or surgery.
Drug manufacturers and healthcare systems could also give more attention to monitoring side effects and dosing. Clinics with good follow-up care see better results. My experience with my friend’s long-term follow-ups showed that frequent check-ins meant problems got spotted faster and adjusted before becoming emergencies. Health teams with pharmacists, nurses, and doctors all pitching in keep folks on track and out of hospital beds.
Final Thoughts
Acetohydroxamic acid shows that even medicines with a niche following can deliver hope to people out of options. Staying informed, keeping a close eye on risks, and building a network around people who need these drugs can make the difference between constant setbacks and a shot at feeling normal again.
What are the possible side effects of Acetohydroxamic Acid?
What Happens Beyond the Basics
I first heard about acetohydroxamic acid in a cramped clinic waiting room, flipping through a medical pamphlet that warned about taking anything without checking the risks. Most folks hear about this medication if they’re tackling chronic urinary tract infections caused by certain types of bacteria. Doctors sometimes reach for it when other antibiotics just don’t cut it. It works by stopping bacteria from making the substances that let kidney stones form, especially in folks already fighting tough infections. But that same pamphlet sat open on my lap longer than most—it didn’t sugarcoat the facts: this drug can bring its own flavor of trouble.
Common Experiences Reported by Real People
Stomach pain and nausea top the charts. Some people feel an urge to throw up not long after taking it, and food doesn’t sit well. I’ve met a few folks in support groups who say having acetohydroxamic acid on board meant learning new bathroom routes. Appetite drops off too. Doctors confirm this isn’t rare, and a study published in the Journal of Urology showed that over 25% of patients reported digestive discomfort within the first few weeks. Sometimes people get a metallic taste in their mouths, and even water can taste off. It’s the kind of side effect you try to ignore but can’t stop noticing.
Less Talked About, More Concerning Effects
Some risks run deeper. Changes in blood count can happen, like anemia or low platelets. The FDA has flagged acetohydroxamic acid as a drug where patients should get blood tests checked regularly. I once shared a coffee shop table with a nurse who saw a patient bruising easily after a few months on the drug—it was a sign of platelets dropping. The impact on the liver matters too. Labs may show liver enzymes rising, hinting that the liver’s struggling to keep up. Headaches, confusion, and even tremors make the list. Anyone with kidney problems walks a tougher path with any medication, and this drug especially needs careful monitoring since it’s cleared from the body through the kidneys.
Why Watchfulness Matters
I’ve always noticed that folks taking medication for months at a stretch start to trust their routines. But with acetohydroxamic acid, changes can creep in quietly. There’s a real need for people to pay attention—from patients to doctors. Doctors at Mayo Clinic tell their patients to look out for yellowing of the eyes or skin and let someone know right away if it shows up. I’ve heard folks brush off fatigue or dizziness, not realizing it’s related to their medication and not just a tough week. I remember a friend mixing this drug with other common medicines, not knowing how it could interact.
Staying Informed, Staying Safe
The best approach leans on strong habits: asking questions, keeping up with bloodwork, knowing what each symptom might mean. Nobody likes talking about side effects, but being quiet about them doesn’t make them go away. Patients do best connecting with pharmacists and doctors who answer honestly. There are no shortcuts to safety, but recognizing how your body feels on a new medication goes a long way. The stories we share help others see that vigilance isn’t fear—it’s good sense.
How should Acetohydroxamic Acid be taken or administered?
Looking at a Lesser-Known Medication
If you’ve heard about acetohydroxamic acid, you probably or maybe know someone who’s struggled with stubborn urinary tract infections, especially those tangled with kidney stones. This isn’t a medicine most people stumble across in their local pharmacy. It’s used in rare cases when standard antibiotics don’t work, mostly in complicated infections where bacteria like Proteus make the urine alkaline and help stones form. My own experience helping a close relative through a challenging infection taught me: the right information on medication use matters as much as the prescription itself.
Getting It Right: Directions Say Everything
Doctors usually prescribe acetohydroxamic acid as an oral tablet. They tell patients to swallow it whole with water, rather than breaking or crushing it. Usually, it goes down one or two times a day, depending on what the lab results say and the patient’s age, kidney health, and other medicines they are already taking. Meals matter. Taking it with food can lower stomach upset, which is a real complaint with this drug.
A typical adult dose hovers around 250mg two to three times daily, though the doctor running the show might adjust that. I can’t stress enough how easy it is to forget a dose or double up without thinking, so setting reminders or using a pill organizer can help keep things on track. Missing doses reduces the drug’s effectiveness, making the infection fight harder and longer. Doubling up can ramp up the side effects, which with this medication can sometimes land a person in the hospital.
Safety Isn’t Optional
This drug isn’t gentle on everyone. Acetohydroxamic acid can hit the kidneys hard, especially in those with weak kidney function to begin with. Blood tests before and during use help spot trouble early; ignoring these checks can end badly. Side effects range from mild—nausea, headaches—to serious ones like blood-clotting problems or mental confusion. Patients should keep the prescribing doctor in the loop about new symptoms, even if they feel small or unrelated.
Mixing medications can turn risky fast. Anticoagulants, antacids, and drugs that alter kidney function might interact badly. That’s why I always suggest carrying an updated medication list to every appointment, so nothing falls through the cracks. Drinking lots of water helps flush bacteria and stone debris, but in people with advanced kidney disease, a doctor’s advice on daily fluid intake comes first.
Who Says No?
Women who are pregnant or breastfeeding shouldn’t take acetohydroxamic acid. Kids under 18 generally avoid it too because there isn’t enough safety data. Allergic reactions—rash, shortness of breath, swelling—signal an immediate stop and a call to the doctor. My personal rule: never downplay new or sudden symptoms on any medication, but especially one with a reputation for tough side effects.
Better Communication, Better Outcomes
I believe straightforward conversations between patients and healthcare teams lead to fewer surprises and less risk. Ask questions, repeat back instructions, and insist on clear explanations especially in high-stakes situations with medications like this. The best results show up in people who know what they’re taking and why, who pay attention to their bodies, and who feel empowered to speak up if something feels off.
Are there any precautions or contraindications for using Acetohydroxamic Acid?
Getting Real About Acetohydroxamic Acid
Acetohydroxamic acid, known in some labs and pharmacies as AHA, steps into the spotlight mainly for treating chronic urinary tract infections—especially those stubborn cases linked to kidney stones caused by urea-splitting bacteria. Folks who have gone through the cycle of recurrent UTIs know how exhausting it feels. The way AHA works—disrupting the enzyme (urease) that certain bacteria use to make stones and ammonia in the urinary tract—offers relief when antibiotics just don’t cut it anymore.
Not a One-Size-Fits-All Remedy
Plenty of people think grabbing a medication is as simple as popping a pill, but AHA reminds us that life isn’t that simple. Strong medicines, especially rare ones like this, often come with a list of people who really shouldn’t touch them. For example, anyone with kidney problems finds themselves in a risky spot. Since the kidneys clear this medication, those with poor kidney function can end up with dangerous buildup in the body. The same goes for folks with liver disease—AHA can mess with the way the liver processes certain substances and might tip the balance in a bad direction.
Main Red Flags to Know
Health authorities and experienced doctors always put blood health near the top of their watch list. AHA can sometimes lead to blood disorders—anemia, low white blood cells, or low platelets. People with pre-existing blood issues, like those fighting off hemolytic anemia, face extra risks since AHA can trigger or worsen these problems. Regular blood tests become a non-negotiable part of treatment for anyone on this drug.
Pregnancy throws up another major stop sign. Data on AHA risk during pregnancy may still be slim, but most health organizations call for great caution. Those planning a family, already expecting, or breastfeeding need a serious sit-down with a medical professional before thinking about AHA. Small children, too—especially those under twelve—should steer clear. Their bodies process medicines differently, and it’s just not worth shooting in the dark with a drug like this.
Drug Interactions and Extra Watch Points
If someone already manages a long list of pills each day, adding AHA makes things complicated. Some meds, like anticoagulants (used to thin the blood), can interact badly. Mixing them can magnify the risk of bruises or major bleeding. The same goes for any drug that’s tough on the kidney or liver. Doctors need a full and honest list of everything a patient takes—not just prescription meds, but over-the-counter remedies and supplements, too.
What to Look Out For: Side Effects and Warning Signs
Everyone expects some side effects from medication, but AHA can hit harder than most. For some, headaches, skin rashes, or stomach issues pop up. More serious signs, like jaundice (yellow skin or eyes), extreme tiredness, shortness of breath, or unexpected bleeding, mean it’s time to seek medical help fast. I’ve known patients get a wakeup call from even a mild rash—they learned it pays to follow up quickly, especially with something this potent.
Better Safe Than Sorry
Doctors and patients both play roles in keeping things safe. No skipping blood tests. Open conversations matter, including honesty about new symptoms, even if they seem small or embarrassing. If in doubt, ask questions before each refill. Meanwhile, health care systems can help by making sure info on rare drugs like AHA flows easily between hospitals, clinics, and pharmacies, so nothing falls through the cracks.
| Names | |
| Preferred IUPAC name | N-hydroxyacetamide |
| Other names |
N-Hydroxyacetamide Acetohydroxyamic acid AHA Acetamidoxime |
| Pronunciation | /ˌæsɪtoʊ.haɪˌdrɒkˈsæmɪk ˈæsɪd/ |
| Preferred IUPAC name | N-hydroxyethanamide |
| Other names |
AHA Acetamide, N-hydroxy- N-Hydroxyacetamide |
| Pronunciation | /əˌsiːtoʊ.haɪˌdrɒksˈæmɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 56-92-8 |
| 3D model (JSmol) | `3d:JSmol/acethydroxamicacid.c3d` |
| Beilstein Reference | 363708 |
| ChEBI | CHEBI:945 |
| ChEMBL | CHEMBL1392 |
| ChemSpider | 11060 |
| DrugBank | DB00551 |
| ECHA InfoCard | 100.041.642 |
| EC Number | 3.5.1.9 |
| Gmelin Reference | 8716 |
| KEGG | C02100 |
| MeSH | D000096 |
| PubChem CID | 528 |
| RTECS number | AF7890000 |
| UNII | P1JM4HV43K |
| UN number | UN3431 |
| CompTox Dashboard (EPA) | DTXSID3023877 |
| CAS Number | 546-88-3 |
| Beilstein Reference | Beilstein 1712057 |
| ChEBI | CHEBI:1140 |
| ChEMBL | CHEMBL1257 |
| ChemSpider | 64269 |
| DrugBank | DB00551 |
| ECHA InfoCard | 100.008.436 |
| EC Number | 211-543-9 |
| Gmelin Reference | 68277 |
| KEGG | C06505 |
| MeSH | D000079 |
| PubChem CID | 8300 |
| RTECS number | AH8225000 |
| UNII | 8TIE4Z7368 |
| UN number | UN3431 |
| CompTox Dashboard (EPA) | DTXSID2021442 |
| Properties | |
| Chemical formula | C2H5NO2 |
| Molar mass | 91.09 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.61 g/cm³ |
| Solubility in water | Soluble |
| log P | -0.48 |
| Vapor pressure | 5.9 x 10^-7 mmHg (25 °C) |
| Acidity (pKa) | 9.34 |
| Basicity (pKb) | 9.34 |
| Magnetic susceptibility (χ) | -47.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.554 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.49 D |
| Chemical formula | C2H5NO2 |
| Molar mass | 74.08 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.61 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.01 |
| Vapor pressure | <1 mm Hg (25°C) |
| Acidity (pKa) | 9.34 |
| Basicity (pKb) | 9.34 |
| Magnetic susceptibility (χ) | -49.0e-6 cm³/mol |
| Refractive index (nD) | 1.468 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.9197 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 116.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -333.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -499.2 kJ/mol |
| Std molar entropy (S⦵298) | 151 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -333.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -515.8 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | J01XX03 |
| ATC code | G04BX03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H301: Toxic if swallowed. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Autoignition temperature | 400 °C |
| Lethal dose or concentration | LD50 oral rat 940 mg/kg |
| LD50 (median dose) | LD50 (median dose): 940 mg/kg (oral, rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 g |
| Main hazards | Toxic if swallowed. Causes severe skin burns and eye damage. May cause allergy or asthma symptoms or breathing difficulties if inhaled. |
| GHS labelling | GHS02, GHS05, GHS07, GHS08 |
| Pictograms | GHS05,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-1-W |
| Autoignition temperature | 400 °C |
| Lethal dose or concentration | LD50 Oral Rat 740 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 720 mg/kg |
| NIOSH | NIOSH: MN1750000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.01 - 0.1 mg/kg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Hydroxylamine Acetamide Hydroxyurea N-Hydroxyphthalimide |
| Related compounds |
Formohydroxamic acid Hydroxylamine Acetone oxime |

