Acetic Acid: A Deep Dive into an Everyday Essential
Historical Development
Acetic acid traces its roots far back, even before chemistry earned its name. Folks stumbled upon it through the souring of wine, realizing that when grapes or grains spoiled, something sharp gave vinegar its punch. By the Middle Ages, alchemists across Europe were boiling vinegar, trying to distill this “spirit of vinegar” into something purer, finding early uses for what they called “acetum.” French scientist Pierre Berthelot and German chemist Hermann Kolbe made great strides in the 19th century: Kolbe synthesized acetic acid from inorganic matter, proving it didn't just have to come from spoiled food. Today, the chemical industry pumps out millions of tons each year, keeping the compound in homes and labs on every continent. The story of acetic acid drips with human curiosity and a drive to use every scrap of what nature offers.
Product Overview
Acetic acid doesn’t need fancy packaging to make a mark. In its dilute form, it turns up as the tang in every bottle of vinegar on grocery shelves. Industry embraces its concentrated form, tossing it around under the label “glacial acetic acid” for all sorts of jobs, from plastics to solvents. It may not draw much attention in the line-up of household chemicals, but behind the scenes, it powers processes in food, textiles, photography, and medicine. Whether packed in a glass jug or squeezed in drums on loading docks, it stays the same clear, pungent liquid that’s saved food from rot and fueled chemical breakthroughs.
Physical & Chemical Properties
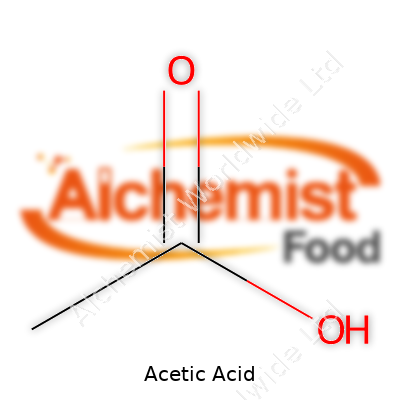
Anyone who’s worked with acetic acid picks up its character fast – that biting smell cuts through the air, hitting eyes and nose long before a drop falls. In its pure state, the liquid looks completely clear. At just above below-room temperature, it starts to freeze, forming solid chunks called “glacial” for a reason. On a molecular level, acetic acid sports a simple structure: two carbons, four hydrogens, and two oxygens. Its acidity measures around pH 2.4 with water, strong enough to pickle cucumbers, not quite so aggressive to chew through metal. Mix it with water, and it blends with no drama, but toss it at strong bases or oxidizers, and a reaction heats up fast.
Technical Specifications & Labeling
Clarity matters in industry, so bottles and drums come marked with purity – usually topping 99% for “glacial” grades. Food-grade versions keep contaminants low, tested batch by batch for the faintest hint of metals or organic leftovers. Safety labels flag its corrosiveness, and transport codes highlight its flammable nature. Labs look for figures like a boiling point of 118°C and a density close to 1.05 g/cm³, no guesswork needed. Labels carry hazard signs with pictograms warning of burns and environmental impact, and documentation runs thick with handling procedures for every imaginable mishap.
Preparation Method
Old vinegar barrels had their day, but modern methods use carbon chemistry to keep the world supplied. Most plants start with methanol, driving it against carbon monoxide in reactors with metal catalysts riding herd on the reaction. This “Monsanto process” knocked out the old natural fermentation for sheer scale and speed. China and a growing list of countries also turn to oxidative methods, starting from acetaldehyde or even directly from ethylene. All routes end up concentrating and purifying the final product, slicing out traces of water, leaving the familiar, nose-tingling acid behind.
Chemical Reactions & Modifications
People often take acetic acid as it comes, but chemistry lets anyone tweak it to suit hundreds of uses. Combine it with alcohols, and esters tumble out – the fruity smells behind nail polish remover and certain artificial flavors. Latch it to fatty acids, and plastics like cellulose acetate step off the production line, spinning into fibers and camera film. It’s a trusty solvent for many organic compounds and offers a straightforward path to vinyl acetate, a key building block in paints and adhesives. Acid chlorides, anhydrides, and salts—turning acetic acid into other chemicals depends on strong hands in a well-ventilated lab, plus careful timing and temperature.
Synonyms & Product Names
The world knows acetic acid by more names than a traveling stage magician. “Ethanoic acid” turns up in some textbooks, reflecting its simple carbon backbone. Pickle factories and home kitchens favor “vinegar acid,” a nod to its source and strength. Industry leans on “glacial acetic acid” for the undiluted version. Chemists rattle off codes like CAS 64-19-7 for paperwork, but on shipping manifests, nothing beats the plain name: acetic acid.
Safety & Operational Standards
Acetic acid can burn the skin or sear lungs if spilled or inhaled, so anyone handling it learns to suit up fast with gloves, goggles, and a sturdy apron. Regulations stick tight, forcing companies to train workers and spell out emergency plans. OSHA sets workplace exposure caps, and the EPA monitors waste, reminding everyone that acid runoff can darken a stream or kill fish downriver. Transport follows the UN’s dangerous goods codes, requiring tight lids, sturdy packaging, and plenty of warning labels. Splash even a small amount on the skin, and medical crews expect redness and pain, so first aid starts with lots of water and quick trips to the nurse.
Application Area
Acetic acid threads through daily life in surprising ways, far beyond kitchen counters. Textile mills rely on it to dye fabrics, shifting colors through precise tweaks in acidity. Farmers treat hay with it to avoid spoilage, keeping feed free of mold. Cleaners swear by its ability to dissolve limescale, while labs use it to fix and preserve biological samples. Pharmaceutical plants lean on acetic acid to produce antibiotics like penicillin, and food makers depend on it for pickles, sauces, salad dressings, and more. Electronics companies and rubber producers each claim a spot in the order books. Its reach quietly shapes comfort, flavor, safety, and scientific discovery in more places than most realize.
Research & Development
Chemists with an eye on sustainability keep chasing new ways to crank out acetic acid with less impact. Bio-based routes turn sugars, wood chips, and even leftover waste into a feedstock for savvy microbes, slashing the carbon footprint compared to fossil sources. Teams look at pulsed electric fields and greener catalysts to improve yields and cut down on byproducts. Some labs chase higher purity, aiming at niche electronics or pharmaceutical uses where a stray ion can wreck weeks of work. There’s also a lively hunt for new acetate-based compounds, especially where materials or medicine need an edge.
Toxicity Research
Acetic acid, diluted, won’t harm more than it helps – vinegar forms a staple for many diets, and low-level exposure raises few health alarms. Ramp up the concentration, though, and risks pile up: burns, corneal damage, and respiratory distress turn up in medical records after misfires or spills in unprepared workplaces. Researchers catalogue these incidents, keeping safety protocols clear and updated. Longer-term studies explore any links between chronic exposure and organ systems, but so far, data suggest acute risks dominate, with good ventilation and protective gear serving as the first and best defense. For the environment, breakdown in water usually runs fast, but industrial spills still force local lockdowns to protect wildlife and water supplies.
Future Prospects
Green technology pushes hard on the world of chemical production, and acetic acid sits in the crosshairs. Designers of biorefineries see it as a gateway compound—produce it from agricultural waste, and the economics of renewable chemicals tilt in their favor. Demand rises hand in hand with plastics and packaging, but regulation means emissions and waste streams need new solutions. Excitement builds for engineered enzymes that produce tailored acetic acid, powering specialty chemicals for medical and electronic uses. From the lab bench to multi-ton reactors, the road ahead depends on finding ways to squeeze more value from every atom while shrinking environmental impact to keep food, industry, and nature in productive harmony.
What are the main uses of acetic acid?
The Role of Acetic Acid in Everyday Life
Most people know acetic acid as the ingredient that gives vinegar its sharp, tangy flavor. A splash in salad dressing, a bite in pickles—without it, so many dishes would taste flat. This simple acid, though, spreads out far beyond kitchen shelves and home remedies.
Acetic Acid in Food and Beverage
You taste it every time you pour vinegar into your cooking. Food producers use acetic acid as both an acidifier and a preservative, holding back spoilage and sharpening flavors. Bottled sauces, condiments, especially in Asian cuisine, and canned vegetables hold up longer because of acetic acid. Bakers turn to it for adjusting dough pH, which affects how bread rises and tastes. Having grown up in a family where pickling cucumbers and onions signaled the end of summer, I’ve seen firsthand how bottled white vinegar—almost pure diluted acetic acid—kept those garden pickles safe from spoilage for months.
Industrial Uses in Plastics and Textiles
Acetic acid is a cornerstone for the chemical industry. Manufacturers turn it into chemicals like vinyl acetate monomer. That’s how you get the building block for polyvinyl acetate, the white glue from back-to-school supply lists, and all sorts of paints and coatings. The fabric on old windbreakers and slick raincoats owes plenty to the acetate made from acetic acid, as does the film on old camera rolls. These uses often feed into products that touch millions of lives without most even realizing the connection to so humble an acid.
Cleaning and Household Applications
Plenty of people swear by vinegar as a natural cleaner, and science backs that up. Acetic acid dissolves limescale, cuts through greases, helps keep glass streak-free. Hospitals and clinics use diluted formulas to disinfect surfaces and instruments. At home, I use it to keep coffee makers clear of residue, and to make hard water stains vanish from bathroom fixtures. Unlike some harsh cleansers, household vinegar generally leaves behind fewer harmful residues.
Medicine and Healthcare
Doctors and dentists both lean on acetic acid. Diluted solutions can stop minor bleeding during procedures. In some clinics, it helps screen for cervical cancer—acetic acid causes abnormal tissues to turn white temporarily under examination. The wound cleaning and mild antibacterial properties highlight its flexible nature, even though no one would call it a cure-all.
Challenges and Safer Use
Industrial-strength acetic acid can burn skin and damage lungs if handled without precautions. News stories have followed cases where mishandling at a plant sent workers to the emergency room. For home users, using household vinegar is generally safe, but eye and skin contact should still be avoided. Education around storage, labeling, and safe dilution protects families and workers alike. Clear labeling and routine worker training go a long way to prevent accidental releases or exposures.
Looking Ahead
With growing interest in more natural preservatives and sustainable products, the demand for acetic acid won't fade soon. Research into bio-based production methods, using waste biomass, might lessen environmental stress from traditional manufacturing. In the meantime, whether on kitchen shelves or chemical plants, acetic acid continues to prove that sometimes the simplest compounds do a world of work.
Is acetic acid safe to handle and use?
Looking at Acetic Acid Up Close
Acetic acid shows up on just about every kitchen shelf as vinegar. It’s easy to forget that this familiar liquid sits in a family of chemicals with a stronger personality in higher concentrations. The kind in vinegar runs around 5%. That’s smooth sailing. The tougher stuff, especially above 10%, deserves a lot more respect.
The Real Risks
Anyone who’s spent time in a chemistry lab or around industrial sites knows strong acetic acid packs a punch. Breathing in those sharp fumes can sting your nose and throat. Splash a high concentration on your skin, and you might feel a burn pretty quick. I’ve seen what a little carelessness can cost: a college lab partner lost their grip on a bottle, got a splash on their arm, and wound up with a nasty red patch that took a few days to calm down. Eyes, as always, face the highest risk. Even a tiny mist can leave you squinting in pain and heading for the eyewash station.
Industrial-grade acetic acid pushes the concerns farther. Higher concentrations carry the risk of fire, along with strong corrosiveness. The Occupational Safety and Health Administration sets the exposure limit at 10 parts per million in the air over an eight-hour shift. Any more, and you risk headaches, coughing, or worse.
Why It Matters
It’s tempting to brush off acetic acid as kitchen-safe after years of using vinegar, but ramp up the strength and you’re in new territory. In concentrated form, this acid moves from salad dressing to something that needs thoughtful handling. Professionals spend years learning how to spot risks and use protection, but I’ve met plenty of people who underestimate what “household” chemicals in laboratories or garages can do.
Looking at the bigger picture, think about all the products where acetic acid plays a quiet role: plastics, solvents, cleaners. Handling it safely keeps home experimenters out of harm’s way and protects workers in factories who rely on the stuff for their paycheck. That matters for anyone who values safe homes and responsible workplaces.
Protecting Yourself with Simple Habits
Gloves, goggles, and a working fume hood go a long way. This comes from spending enough hours in both high school and university labs, where safety checks mattered more than speed. One forgotten pair of safety goggles or one ignored warning sign sets you up for the kind of day you don’t want to repeat. If you need to work with stronger acetic acid at home, make sure to have a well-ventilated space and keep neutralizing agents like sodium bicarbonate nearby. Even in a pinch, it’s better to call for help instead of pushing through with bad air or exposed skin.
For those who spend their days in industries that use acetic acid, proper storage can make a difference. Tight seals, labeled containers, and clear protocols for spills don’t just tick boxes—they help people go home safe. Companies have a role to play by giving folks real training, not just a checklist. None of this takes away the usefulness of acetic acid, but it shapes how we use it without risking health and livelihoods.
Sharing stories and facts about acetic acid isn’t meant to scare, but to keep things real. Respecting hazards doesn’t mean avoiding a useful tool; it just means giving it the attention it’s earned through experience.
What is the concentration of acetic acid in this product?
Why Knowing the Amount Matters in Everyday Products
Acetic acid crops up in all sorts of everyday products, from vinegar on a salad to the strong-smelling cleaner in the cupboard. Most folks rarely stop to check just how much acetic acid a bottle of vinegar has, but the amount makes a difference. Consuming diluted vinegar gives a tang to food and helps preserve pickles. A stronger solution cleans hard water stains or unclogs a coffee maker. Both use cases need knowledge and care — and not knowing the concentration can waste money or even be unsafe.
How Concentration Levels Affect Use and Safety
Walk down a grocery aisle and you’ll see bottles saying “5% acidity.” That percentage describes grams of acetic acid in 100 milliliters of liquid. Most table vinegars stick right around that 5% mark. It adds flavor without burning your tongue or the inside of your throat. Stronger vinegar, used for cleaning, can crank that number up to about 20%. Anything above that could cause chemical burns if spilled on skin or splash in the eye. So even something as familiar as vinegar holds potential for misuse if the label gets ignored.
Putting this into perspective, researchers and safety guidelines back these limits for good reason. According to food safety regulations in the United States, only vinegar with an acetic acid content between 4% and 8% is considered safe to eat or cook with. There isn’t much room for guessing games. Food producers and cleaners need solid testing, especially with homemade or imported bottles showing up at local markets.
Testing Methods Used in Real Life
Simple acid titration stands as the gold standard for measuring acidity. I remember my first chemistry experiment—measuring the acid in a mystery liquid using nothing but a burette, some colored indicator, and cautious hands. It demands patience and a sharp eye, but delivers trustable results. Big producers rely on automated tests, using machines that churn through hundreds of samples for quality control.
Home cooks can trust the label on store-bought vinegar. Small-scale makers selling at farmer’s markets sometimes get batches tested at independent labs. Most authorities urge anyone making concentrated cleaners or medicinal vinegars at home to do so with clear knowledge of the risks and limits. Neglecting concentration checks opens the door to burns, ruined recipes, and failed food safety inspections.
Keeping Everyone Safe and Informed
Labels and honest information matter. Confusion about acetic acid percentages leads to accidents or shoddy results. I’ve seen folks clean with the same vinegar they drizzle over lettuce, wondering why it’s not working well on limescale. Strong cleaners labeled as “industrial” sometimes don’t make it clear just how potent they are until someone finds out the hard way. Clear, readable labeling and plain language about intended use would mean fewer calls to poison hotlines and far fewer kitchen mishaps.
Public health relies on everyone getting the right facts. Whether making salad or scrubbing grout, the concentration makes a world of difference. Basic awareness, careful labeling, and accessible testing give everyone more control and better results.
How should acetic acid be stored?
Why Proper Storage Matters
I remember the first time I worked with acetic acid in a lab. The strong smell hit me long before I ever poured it. Most of us know acetic acid as the main ingredient in vinegar but in concentrated forms, it can burn your skin, damage equipment, and even spark a fire. So, proper storage isn’t just a suggestion. It helps protect people and property—and keeps lab work running safely.
Basic Rules That Pay Off
Glass or high-density polyethylene containers work best. Early in my career, I learned to avoid metal, especially iron and steel, since acetic acid loves to eat those for breakfast and leave you with leaks. Glass gives peace of mind because it doesn't react and you can easily check what’s inside. High-density polyethylene also stands up to strong acids and gets used across labs for that reason.
Don't ever store acetic acid in open containers or near vents. Strong fumes can escape—making the air inside a workspace harsh, and in high concentrations, flat-out dangerous. Always make sure lids fit tight. Spills can do real damage, both to people and lab benches—just a quick brush of skin brings painful burns, and nobody wants those kinds of surprises.
Temperature and Location Count
Acetic acid’s flash point is low—39°C (102°F)—so storing it near heat sources, water heaters, or even a spot in the sun just isn’t smart. Safety manuals draw hard lines on this. A cool, well-ventilated chemicals cabinet, kept out of direct sunlight or any sort of heat, keeps risks low. At the university, we kept ours in flame-proof cabinets. It felt a little extra, but if a fire breaks out, you want acetic acid locked away, not fueling the flames.
Humidity creates another headache. The acid can absorb water from the air over time, so air-tight containers matter. Over time, moisture can dilute the acid, and you lose track of how strong your substance is—which can ruin experiments and pose safety risks.
Keep It Isolated
Acetic acid doesn’t mix well with oxidizers, including bleach and hydrogen peroxide. Mixing these creates potent, dangerous reactions—sometimes explosive. Separate shelves, well-labelled and monitored, make sure everyone stays out of trouble. At my old lab, the safety officer laid down red tape—literally—and nothing crossed that line unless it belonged there.
Clear labels stand as your first line of defense against accidents. I’ve seen too many rushed researchers pour from one bottle to another and forget which is which. Mark containers with the name, concentration, and hazard warnings. Run regular checks. Everyone slips up sometime but a good label keeps mistakes from turning bad.
Personal Experience: Mistakes Happen Fast
One accident sticks with me—a co-worker spilled a beaker. Since he wore only latex gloves, rather than thicker nitrile ones, the acid cut through in seconds. Quick action with the safety shower prevented scarring but it could have been much worse. Gloves, goggles, and lab coats are worth every second they take to put on.
Solutions That Work
Get everyone trained—don’t just hand them the safety data sheet and hope for the best. A clear walkthrough, maybe even a yearly refresher for long-timers, keeps safety on everyone’s radar. Invest in cabinets built for chemical storage. They cost more upfront but cut down on insurance claims, injuries, and lost time. Audit your setup once a season; problems creep in as supplies are used and replaced.
Safety supervisors, clear signage, and emergency wash stations make storage failures less deadly. With simple habits and the right gear, storing acetic acid becomes routine—and feeling safe at work improves the whole atmosphere.
What precautions should be taken when using acetic acid?
Understanding Acetic Acid and Its Risks
Acetic acid shows up in plenty of places we might not expect. Most folks know it as the main ingredient in vinegar, but higher concentrations turn it from a kitchen staple into a powerful solvent and chemical that can harm skin, eyes, lungs, and even our internal organs. In labs and industrial spaces, acetic acid usually comes strongly concentrated, so one small splash or whiff can cause big trouble. As someone who has handled chemicals for both school work and DIY projects, I learned the hard way that shortcutting on safety doesn’t just risk your health — it can create chaos for everyone around you.
Personal Protection Comes First
Before even opening a bottle of acetic acid, the right personal protection can mean the difference between a normal day and a trip to urgent care. Nitrile or neoprene gloves form a reliable barrier because acids chew through ordinary latex. Safety goggles matter more than people realize, since acid fumes and droplets can destroy the thin membrane over your eyes. A face shield adds another layer if there’s risk of splashing. Always choose a sturdy lab coat or chemical apron, since cotton and synthetics can soak up spills without offering much resistance. For anyone with facial hair like me, a snug mask gives some extra reassurance against fumes slipping through gaps.
The Importance of Airflow
Good ventilation isn’t just a recommendation. Acetic acid’s vapors sting your nose and throat, and in high concentrations, can scar your airway. In my own work, I stick to a fume hood or at least a space with strong cross-breezes and an open window. Building up fumes indoors courts disaster for everyone nearby. Even if the smell doesn’t feel strong to you, low-level exposure can cause headaches and breathing problems that creep up unexpectedly. Making sure the area stays clear of bystanders — especially kids and pets — gives peace of mind.
Storage Mistakes Can Haunt You
Leaving chemicals out in the open or near reactive items never ends well. Acetic acid belongs in sturdy, labeled containers, away from sources of heat and sunlight. Acid and strong bases have a nasty habit of reacting explosively, so shelves should never mix the two. I’ve seen friends overlook this warning and lose not just their materials, but also trash a refrigerator or cupboard. Keeping emergency spill kits and neutralizing agents — like baking soda or calcium carbonate — within arm’s reach can save your workspace and even your hands in a pinch.
Boring but Crucial: Reading Labels and Directions
Many people feel tempted to skip reading labels, trusting their memory—especially during routine work. That’s a mistake. Manufacturers often put essential, unique tips on handling and mixing, especially for concentrated solutions. The type of container matters, too; metal and glass react differently to acid. My advice is to read twice, open once, every single time you’re about to pour or dilute.
No Substitute for Quick Cleanup and Emergency Plans
Even the most careful person can spill or splash, so having clear cleanup procedures makes all the difference. Water quickly dilutes acetic acid, but it spreads the spill if you’re not careful. Absorbent pads, neutralizing powders, and clear exits create a safety net if things go sideways. Posting emergency numbers by your workspace and rehearsing what to do in a crisis build confidence under stress, which I’ve appreciated during more than one near miss.
Takeaway: Respect, Don’t Fear
Knowing the dangers and showing acetic acid respect helps prevent injuries and stress in the long run. Personal stories from professionals and hobbyists alike show that a few extra minutes setting up safe practices pay off every single time, whether you’re working in a bustling lab or just unclogging a drain at home.
| Names | |
| Preferred IUPAC name | ethanoic acid |
| Other names |
Ethanoic acid Vinegar acid Methanecarboxylic acid |
| Pronunciation | /əˈsiː.tɪk ˈæs.ɪd/ |
| Preferred IUPAC name | ethanoic acid |
| Other names |
Ethanoic acid Vinegar acid Methanecarboxylic acid |
| Pronunciation | /əˈsiː.tɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 64-19-7 |
| Beilstein Reference | Beilstein Reference: 1718732 |
| ChEBI | CHEBI:15366 |
| ChEMBL | CHEMBL: CHEMBL153 |
| ChemSpider | 175 |
| DrugBank | DB03166 |
| ECHA InfoCard | 100.003.284 |
| EC Number | 200-580-7 |
| Gmelin Reference | 635 |
| KEGG | C00033 |
| MeSH | D001010 |
| PubChem CID | 176 |
| RTECS number | AF1225000 |
| UNII | Q369O8926L |
| UN number | UN2789 |
| CompTox Dashboard (EPA) | DTXSID2029074 |
| CAS Number | 64-19-7 |
| Beilstein Reference | Beilstein Reference: 1718733 |
| ChEBI | CHEBI:15366 |
| ChEMBL | CHEMBL: CHEMBL715 |
| ChemSpider | 174 |
| DrugBank | DB03166 |
| ECHA InfoCard | 03-2119471839-32-0000 |
| EC Number | 200-580-7 |
| Gmelin Reference | 162 |
| KEGG | C00033 |
| MeSH | D001650 |
| PubChem CID | 176 |
| RTECS number | AF1225000 |
| UNII | Q369O3754G |
| UN number | UN2789 |
| Properties | |
| Chemical formula | C2H4O2 |
| Molar mass | 60.05 g/mol |
| Appearance | Clear, colorless liquid with a pungent, vinegar-like odor. |
| Odor | Pungent |
| Density | 1.049 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.17 |
| Vapor pressure | 1.5 kPa (at 20 °C) |
| Acidity (pKa) | 4.76 |
| Basicity (pKb) | 14.75 |
| Magnetic susceptibility (χ) | χ = −5.8×10⁻⁶ |
| Refractive index (nD) | 1.371 |
| Viscosity | 1.22 mPa·s (at 25 °C) |
| Dipole moment | 1.74 D |
| Chemical formula | C2H4O2 |
| Molar mass | 60.05 g/mol |
| Appearance | Clear, colorless liquid with a pungent, vinegar-like odor |
| Odor | Pungent, vinegar-like |
| Density | 1.049 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.17 |
| Vapor pressure | 1.5 mmHg (20°C) |
| Acidity (pKa) | 4.76 |
| Basicity (pKb) | 14.76 |
| Magnetic susceptibility (χ) | -5.8×10⁻⁶ |
| Refractive index (nD) | 1.371 |
| Viscosity | 1.22 mPa·s (at 25 °C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 159.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −484.5 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | −875.8 kJ·mol⁻¹ |
| Std molar entropy (S⦵298) | 159.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −484.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –874 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A01AB02 |
| ATC code | A01AB04 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02, GHS05 |
| Signal word | Danger |
| Hazard statements | H226, H314, H318 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P363, P370+P378, P403+P233, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-2-Acid |
| Flash point | 39 °C |
| Autoignition temperature | 463°C |
| Explosive limits | 4% - 19.9% |
| Lethal dose or concentration | LD50 oral rat 3,310 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3,310 mg/kg (rat, oral) |
| NIOSH | KW2625000 |
| PEL (Permissible) | 10 ppm |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | 50 ppm |
| GHS labelling | **GHS02, GHS05, GHS07** |
| Pictograms | GHS02, GHS05, GHS07 |
| Signal word | Danger |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P260, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P363, P370+P378, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-2 |
| Flash point | 39 °C (102 °F) |
| Autoignition temperature | 463°C |
| Explosive limits | 4% - 19.9% |
| Lethal dose or concentration | LD50 oral rat 3,310 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3,310 mg/kg (oral, rat) |
| NIOSH | AS2475000 |
| PEL (Permissible) | 10 ppm |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | 50 ppm |
| Related compounds | |
| Related compounds |
Acetic anhydride Acetyl chloride Ethyl acetate Formic acid Propionic acid Lactic acid |
| Related compounds |
Formic acid Propionic acid Lactic acid Acetyl chloride Acetic anhydride Sodium acetate Ethyl acetate |

