4-Hexylresorcinol: Past, Present, and Future in Science and Industry
Historical Development
4-Hexylresorcinol has a story that winds its roots through both medicine and industrial chemistry. Early research in the 1920s began exploring its antiseptic qualities. Its antiseptic attributes drew the attention of pharmaceutical formulators, as new antibacterial compounds were in high demand. Over time, its use expanded well beyond the laboratory, finding favor in topical treatments, oral care products, and the food industry. Each application sprang out of persistent investigation into phenolic compounds and their effects on both microbes and human tissues. With antibiotics just finding their feet, scientists and manufacturers turned to phenolic derivatives to tackle infection and preserve products before refrigeration was common. This led to 4-hexylresorcinol’s adoption as an anti-microbial in everything from lozenges to seafood processing. Decades later, researchers hit upon new uses, advancing both food safety and cosmetic science with this same molecule.
Product Overview
The reach of 4-hexylresorcinol covers more ground than many expect. This single aromatic compound pops up in throat lozenges, skincare formulations, and shellfish preservatives. Its popularity comes from its power to limit bacterial and enzymatic deterioration in food, as well as its utility in suppressing discoloration in shrimp and other seafood. On the skin, it often appears in products aiming to lighten hyperpigmentation and provide antioxidant protection. The versatility of this chemical lets it cross boundaries, connecting industries like food processing, pharmaceuticals, and personal care. The market’s broad use cases spring from decades of R&D, often driven by a basic truth—4-hexylresorcinol gets the job done where fewer tailored options exist.
Physical & Chemical Properties
In its raw form, 4-hexylresorcinol forms off-white crystals with a faint odor, melting sharply around 70–74°C. It dissolves easily in organic solvents like ethanol, benzene, and ether but resists dissolving in cold water. The compound’s stability makes it valuable, especially since it holds up under regular storage conditions and doesn’t break down when exposed to modest light or air. With a molecular formula of C12H18O2 and a structure built on a resorcinol core capped with a straight six-carbon chain, the molecule packs both hydrophobic and hydrophilic features. This lets it interact with bacterial cell membranes and enzyme systems, providing its famed antimicrobial punch. Such properties are not exotic or rare in chemical taxonomy, but the balance found in 4-hexylresorcinol made it more practical than alternatives for food safety and drug delivery tasks.
Technical Specifications & Labeling
Manufacturers typically supply technical grades of 4-hexylresorcinol in packages that highlight high purity, often above 98%. Labels focus on exact melting points, batch numbers, and compliance with major pharmacopoeias. Food-grade and pharmaceutical-grade lots need documentation tracing product origin, storage conditions, and handling protocols. Clear hazard warnings also appear because this compound, while effective, demands respect and careful handling. Product datasheets pile up detailed analytical reports, including HPLC, IR, and NMR spectra, to build trust with buyers in regulated industries. Lots aimed at drug and food production must pass microscopic quality controls and display complete regulatory details to harmonize with local and international codes.
Preparation Method
Industrial production leans on well-worn organic reactions. Chemists introduce hexyl groups onto the resorcinol backbone through alkylation reactions involving hexyl halides and basic catalysts. Reaction conditions favor high yields and few side products, but careful monitoring keeps the purity above threshold limits for consumer safety. Early chemical texts describe similar procedures, though modern refinements focus on greener solvents, better heat transfer, and lower byproduct waste. Some laboratories play with enzyme-catalyzed steps or switch to microwaves to trim reaction times and push sustainable practices forward. The scalable simplicity of this process helps keep costs manageable for food and drug sectors relying on consistent supply chains.
Chemical Reactions & Modifications
4-Hexylresorcinol stands up well to mild temperatures and oxidizing agents, but strong acids or bases can pry apart the aromatic ring, leading to decomposition and loss of function. Nitration and halogenation are both possible, letting chemists build analogs or test structure-activity relationships in therapeutics and biochemistry. Laboratories also use it as a starting point for more complex molecules, etherification, or esterification. Scientists often study its chemical tweaks for boosting water solubility or altering its antimicrobial footprint. This search for new derivatives speaks to the larger push for multifunctional compounds in medical and industrial chemistry, and to the fact that even proven ingredients get revisited with modern know-how.
Synonyms & Product Names
The chemical registry lists names like 4-n-hexylresorcinol, 4-HR, p-hexylresorcinol, and 4-(Hexyloxy)benzene-1,3-diol. Commercially, you’ll spot it marketed in personal care land as a brightening ingredient for serums and creams. You’ll also see references to CI 60760 or various catalog codes on bulk listings for science education kits and industrial sales. Multiple synonyms and labels often confuse buyers, so technical documentation remains critical to proper sourcing.
Safety & Operational Standards
Regulatory authorities pay close attention to its handling and exposure risks. 4-Hexylresorcinol needs gloves and ventilation during weighing and mixing, as it causes irritation to mucous membranes, eyes, and skin in concentrated forms. Dust controls and spill procedures aim to protect workers, especially in food processing plants where powders travel through the air. Food authorities across continents set tight limits on permissible concentrations in seafood and food packaging. Storage stays straightforward: sealed containers, cool, dry shelves, and clear hazard labeling. In cosmetic applications, testing determines safe thresholds to fend off sensitization and unwanted reactions. Standard operating practices adopted from chemical hygiene plans keep workplaces compliant and staff healthy.
Application Area
Applications range from the familiar—like inclusion in throat lozenges for sore throats—right through to specialized use in shrimp farming and seafood packaging. Food technologists deploy it to curb melanosis or “black spot” in crustaceans after harvest. In cosmetics, it targets excess melanin and delivers mild antioxidant benefits, featuring prominently in formulas branded for Asian markets where lighter, more even-toned skin drives product development. In research, 4-hexylresorcinol frequently appears in enzyme inhibition assays, often as a tool molecule for unpicking reaction mechanisms and biofilm formation in bacteria. Its adaptability helps answer real-world quality and safety challenges, especially when long distance food logistics or product shelf-life are on the line.
Research & Development
The science never really stands still for this compound. Academic papers keep testing its anti-inflammatory, antimicrobial, and enzyme-inhibiting activity, looking to stretch its boundaries into new drug leads or biomaterial coatings. Research groups also probe its role in targeting particular bacteria in dental products without breeding resistance—a problem that stumps many established antibiotics. Food scientists test combinations of 4-hexylresorcinol with natural extracts to create hybrid preservatives that answer a rising demand for cleaner ingredient labels. Each year, new studies chart improved synthesis methods, deeper mechanistic insights, and better application data for both industrial and medical fields.
Toxicity Research
Toxicologists ran controlled studies since the middle of the last century. They determined effective concentrations for antimicrobial activity alongside safe exposure levels for skin and mucosal contact. At low concentrations, 4-hexylresorcinol shows limited adverse effects, although high doses can cause skin irritation, headaches, or mild systemic toxicity. Scientists repeatedly test metabolites and breakdown products to rule out long-term effects or environmental build-up. Food regulators respond to this data, adjusting allowable usage and enforcing strict post-market surveillance to safeguard consumers. In essence, responsible use relies on up-to-date toxicology and honest reporting from end-users in every sector.
Future Prospects
The demands of global supply chains, persistent food waste, and the push for sustainable preservatives sharpen focus on multifaceted compounds like 4-hexylresorcinol. It’ll play a part in cleaner-label foods, smarter packaging, and advanced skincare lines. The march of regulations may shape new guidelines, particularly as consumer groups keep asking how their household products affect health and ecosystems. As more research links microbial balance to holistic wellbeing, this old molecule could take a fresh spot in oral care, dermatology, or even functional foods. The blend of heritage, scientific verification, and commercial relevance makes it a perennial candidate for both fine-tuning existing technologies and breaking into new application fields.
What is 4-Hexylresorcinol used for?
An Additive With Many Roles
Walking through a grocery store, it’s easy to pass by shelves stacked with shrimp, fresh-cut apples, or a mouthwash bottle and not think much about what keeps these products looking good or working well. I’ve spent time working in the food industry, so I notice small details like the use of preservatives to keep food fresh and safe. One of those is 4-hexylresorcinol. It’s not a household word, yet it touches more lives than most people realize.
Keeping Food Looking Good
Seafood, especially shrimp, tends to turn black quickly after harvest. Chemists saw this problem and went after a solution that wouldn’t scare off buyers with a long list of hard-to-pronounce chemicals. 4-Hexylresorcinol came into play because it stops a natural enzyme in shrimp and shellfish from causing melanosis—a fancy term for unsightly black spots. Without it, seafood loses that appealing pink-and-white look so fast that supermarkets could not keep up. FDA says it’s safe in these applications at low concentrations, and I know from experience that food safety teams monitor these levels tightly.
Mouthwash, Throat Lozenges, and Beyond
This compound does more than preserve food. Walk into any pharmacy, reach for products that promise a cleaner mouth or relief from a sore throat, and there’s a strong chance you’ll find 4-hexylresorcinol on the ingredient list. It has antiseptic qualities, so it’s one of the go-to choices for fighting off germs in oral care products. That’s based on years of research showing it slows the growth of bacteria that cause sore throats and mouth infections, which I’ve seen firsthand work better than old-fashioned home remedies.
Cosmetic Science and Skincare
The world of skincare is hard to navigate, with new ingredients popping up all the time. Dermatologists and researchers point out that 4-hexylresorcinol offers more than just surface-level benefits. It helps lighten skin by reducing melanin production, which is why it shows up in some creams aimed at people with dark spots or uneven skin tone. Not only does the science back that up, but the American Academy of Dermatology also lists it as a viable alternative to hydroquinone, which comes with safety concerns for some users.
Thinking About Risks and Responsible Use
Nothing comes free of risk, and this preservative is no different. Food experts, toxicologists, and watchdog agencies agree that high doses could pose health problems, so regulatory bodies like the FDA and EFSA keep strict thresholds, and manufacturers sample their foods often to make sure they stay within limits. I’ve talked with seafood processors who take pride in staying under these caps, not just because it’s law, but because it keeps trust with buyers and their own families who eat the same shrimp.
Better Solutions for the Future
Greater consumer awareness pushes companies to look for cleaner and greener preservatives, but right now, few options work as well as 4-hexylresorcinol in seafood and oral care. I’ve seen labs experimenting with natural extracts like rosemary and green tea, but they can’t match the power or reliability of this established ingredient so far. If the industry wants to move beyond synthetic additives, a lot more investment is needed in R&D to help fresh food and effective products stay affordable and safe on shelves.
Is 4-Hexylresorcinol safe for skin applications?
Understanding 4-Hexylresorcinol in Skincare
4-Hexylresorcinol has started showing up in skin care labels, especially in products claiming a brightening effect. A close look at any bottle with even a whiff of promise for reducing dark spots or evening out skin tone might feature this name tucked near the end of the ingredients list. For those who have sensitive skin or allergies, any new chemical name can raise a few eyebrows.
Real-World Experience and Scientific Evidence
About a year ago, I tried a cream that boasted 4-Hexylresorcinol near the top of its formula. After weeks of use, it seemed kinder to my skin than more aggressive options like hydroquinone. Rather than flakes or burning, my skin just looked a little clearer—no major irritation.
Plenty of reputable journals back up this milder reputation. For over a decade, dermatologists in Asia and Europe have looked at it as an alternative to harsher melanin-inhibitors. Research points out that at typical concentrations (usually around 0.5%), people rarely report redness or other side effects. In repeated patch tests, only a small handful of people had mild irritation. Also, its use in throat lozenges since the 1930s adds a long trail of human exposure, giving us extra real-life data not every skincare ingredient can claim.
Looking at the Pros and Cons
On the plus side, 4-Hexylresorcinol works by targeting tyrosinase, the enzyme that helps produce melanin. Many consumers searching for a more even complexion turn to this sort of ingredient in hopes of fading age spots or acne marks. Some studies even compare it favorably to kojic acid and arbutin, but with fewer reports of sensitization. People with stubborn hyperpigmentation know how frustrating it can be to cycle through product after product, each one promising but not delivering. Adding something that is less likely to trigger irritation is a clear benefit.
No ingredient comes with zero risks. Cases of allergic reaction can still pop up, and not every study out there has checked long-term effects over decades. Manufacturers don’t always guarantee purity or control the percentage perfectly, either. Consumers want to know companies are transparent about sourcing and formulation. Like with any ingredient, mislabeling or excessive concentrations can cause trouble, so sticking to reputable brands is practical.
Stronger Oversight and Consumer Trust
Dermatologists keep close tabs on new data about ingredients like 4-Hexylresorcinol. In the European Union, rules tend to be tight—health authorities have set upper limits for concentrations used in cosmetics. The US FDA has not issued specific warnings, but smart shoppers check for certifications and third-party testing. Skin safety doesn’t depend just on what the label says; it’s about the habit of testing a small patch on your arm before going all in.
Building more trust means more clinical trials, open discussions about rare reactions, and clear labeling about origin and concentration. Those chasing smooth, clear skin deserve the full picture. Giving people enough information and safe options makes better skin decisions possible for everyone.
What are the side effects of 4-Hexylresorcinol?
The Basics of 4-Hexylresorcinol
4-Hexylresorcinol pops up a lot in everyday life, even outside of science labs. It goes into some skin creams, is used as a preservative in foods like shrimp, and shows up in some oral care products. Manufacturers like it because it helps stop discoloration in food and helps keep bacteria in check. What starts out as a practical ingredient often leads to a mix of benefits and concerns for people who end up using it.
Known Side Effects and Concerns
Dermatologists sometimes mention mild skin irritation as a possible downside when 4-Hexylresorcinol is found in skincare. Picture the familiar experience of itchiness or redness after applying a new lotion or cream. Skin sensitivity can depend on personal factors, such as allergies or current skin conditions. The risk ramps up for folks with naturally sensitive or reactive skin types. Those prone to eczema or rosacea notice flare-ups more readily than others.
Some people notice mild stinging when products containing this compound touch mucous membranes—like the lining of the mouth in certain throat lozenges. In my experience, anyone who has dealt with canker sores or mouth ulcers ends up feeling any new ingredient more acutely, and 4-Hexylresorcinol is no exception. Usually these sensations pass quickly, but they stick out as moments people remember, especially if the mouth is already sore.
Looking at food preservatives, research suggests that 4-Hexylresorcinol used in shrimp and shellfish doesn’t build up to high levels in most diets. Regulatory agencies such as the FDA approve its use within strict limits. Even with these controls, a small slice of people experience allergic-type reactions, including hives or swelling, after eating seafood treated with this compound. This runs along the same lines as other food additives and makes label-reading important for consumers with many sensitivities.
Long-Term Health and Research
For years, most studies on 4-Hexylresorcinol’s safety looked at large batches and focused on short-term exposure. Sometimes, people wonder whether low doses in food and cosmetics add up over time. There isn’t strong evidence linking standard use to major health problems, but the research field still has gaps. Animal studies haven’t shown cancer risk or organ toxicity at typical exposure levels, and the World Health Organization sets daily intake guidelines that offer a margin of safety.
People often share stories with each other about how certain chemicals left them feeling “off,” even when studies don’t always line up the same way. It helps when companies study how these ingredients act in diverse real-world settings, instead of only inside labs.
Better Choices and Solutions
Choosing products with 4-Hexylresorcinol comes down to personal health, allergies, and trust in transparency from brands. If you know you react to preservatives or get rashes from new skincare, talking directly to a doctor or pharmacist brings peace of mind. Patch testing new products before using them widely can spare a lot of discomfort for those with unpredictable skin.
Food safety involves reading ingredient lists and asking questions at the seafood counter, especially for people with histories of food allergy. Open conversations with manufacturers and updated scientific reviews help keep everyone better informed. Consumer advocacy has played a big role in pushing for clearer labeling.
The more companies share about safety testing and real user experiences, the more trust people give to products that use this ingredient. It helps to keep up with the latest information and share personal reactions with healthcare professionals, helping future research build on real stories.
How does 4-Hexylresorcinol work as a skin lightening agent?
The Real Power Behind 4-Hexylresorcinol
Plenty of creams and serums out there claim to give brighter skin, but not many ingredients spark as much curiosity as 4-hexylresorcinol. Dermatologists often look for options that safely tackle uneven tone, so learning about this compound matters to anyone facing stubborn hyperpigmentation. Unlike some loud newcomers, 4-hexylresorcinol takes on dark spots at a root level. I spent years trying products that promised glow but left my skin irritated, so finding out how this ingredient works made me rethink skin care with a science-backed lens.
Blocking the Engine of Melanin
This ingredient doesn’t just skim the surface. It goes deeper and blocks tyrosinase, the enzyme in skin cells that sparks melanin production after sun, irritation, or injury. Tyrosinase acts like an on-switch for pigment, and when triggered too much, brown spots get darker. I’ve seen research showing 4-hexylresorcinol slows this pattern. And it’s not just theory—studies published in top dermatology journals point out that it cuts enzymatic activity more sharply than old-school skin lighteners like hydroquinone or arbutin, and with fewer harsh side effects.
Why Pick 4-Hexylresorcinol Over Others?
Some folks stick with hydroquinone, but complaints pile up about dryness and irritation after daily use. Other common lighteners like kojic acid often fall short in either real results or gentle feeling. 4-hexylresorcinol impressed even picky experts at the US Food and Drug Administration for its strong antioxidant kick—helping defend skin from further pigment triggers, like pollution or inflammation. Skin doctors I trust choose this molecule for clients with sensitive skin who struggle after trying harsher treatments.
Safety Stands Out
Any product that interrupts melanin needs solid proof it won't cause chaos in healthy cells. At a time when skin bleaching scandals pop up everywhere, nobody wants to roll the dice on something shady. Multiple studies from the last five years back up its clean track record: no hormone disruption, no DNA damage, and fewer negative reactions compared to traditional lighteners. Every expert worth their salt values this, especially those working with patients of color who carry higher risk for post-inflammatory pigmentation.
Questions in Real-World Results
People often ask how fast they’ll see change. My own experience and what I hear from seasoned estheticians lines up—a few weeks for subtle brightening, with real shift after two or three months if used regularly. This slower improvement beats sudden, severe bleaching that leaves skin patchy. But too many people expect miracle changes overnight and get disappointed. Setting honest expectations counts just as much as the right science.
What Still Needs Fixing?
Transparency remains a huge need. Many products bury their real concentrations of 4-hexylresorcinol behind flashy claims, making it tough for consumers to know what they’re really buying. Brands must share clear details about sourcing and potency. Pricing is another issue—effective products can run pricey, locking out buyers who need affordable options for routine care. Dermatologists and regulators ought to push companies for both honesty and accessibility so users don’t get left behind for wanting healthier skin.
The Path Forward
Education from trusted professionals shapes how people view ingredient safety and results. Science supports using 4-hexylresorcinol for those hoping to fade dark patches without risking long-term harm. Knowledge empowers both patients and the industry. By demanding transparency, fair access, and more robust research, consumers and caregivers alike can move past old myths and chase real, lasting change for all skin types.
Can 4-Hexylresorcinol be used with other skincare ingredients?
Looking Beyond the Hype
4-Hexylresorcinol has become a popular ingredient in skincare, especially among those trying to tackle uneven skin tone, signs of aging, and dullness. Dermatologists use it for good reasons. This molecule carries impressive antioxidant properties and helps block the formation of melanin, which makes it a favorite in brightening serums. The question most people ask is whether it works safely alongside other ingredients. Thinking about what goes on your skin before you mix products is a smart move, since poorly chosen combinations sometimes lead to redness, irritation, or just wasted time and money.
Ingredient Combinations Worth Exploring
Most people using 4-Hexylresorcinol want results. Combining it wisely helps chase stubborn pigmentation or fine lines more effectively. Using it with gentle brighteners like niacinamide, vitamin C, or licorice extract tends to boost the benefits without putting the skin at risk. Niacinamide, for instance, supports the skin barrier and addresses inflammation. My personal experience mixing niacinamide with pigment-correctors like 4-Hexylresorcinol leaves the skin less reactive, especially after a stretch of cold weather or extra mask-wearing days.
Glycolic or lactic acid also play nicely with 4-Hexylresorcinol, as long as you don't pile on too many strong products at once. Acids help exfoliate dead cells, so the pigment-blocking action gets deeper access to skin layers where it counts. One routine that worked well in real life involved alternating glycolic acid pads and a serum featuring 4-Hexylresorcinol, used at different times of the day. Most people don’t see flushing or peeling, which is a relief for anyone with sensitive skin.
Pairing with Retinoids and Sunscreen
Retinoids bring their own legacy for softening fine lines and balancing pigment. If you use both a retinoid and 4-Hexylresorcinol, spacing them apart often avoids irritation. Night routines can include a retinoid, reserving 4-Hexylresorcinol for morning, always paired with a solid sunscreen. Sunscreen matters more than ever with pigment-targeting ingredients because freshly uncovered skin needs protection from UV rays. Not everyone remembers that part, but skipping it can erase months of progress or even trigger rebound pigmentation.
Some people worry if mixing antioxidant serums—like those with vitamin C or E—lessens the punch of 4-Hexylresorcinol. Research supports combining these since antioxidants often complement each other, guarding skin cells on multiple fronts. Take it from those who’ve managed melasma or post-inflammatory hyperpigmentation: using packages that blend several antioxidants with 4-Hexylresorcinol feels gentler and often works better than one hero ingredient alone.
Red Flags and Things to Avoid
Certain ingredients just don’t play well together. Skip formulations with harsh scrubs, pure essential oils, or strong alcohols alongside 4-Hexylresorcinol. Harsh exfoliants may strip the skin and make irritation more likely, especially if your routine already features acids or retinoids. Anyone who has accidentally doubled up on peels and brighteners can recall the persistent redness that follows—a lesson most learn the hard way.
For people with sensitive or compromised skin, patch tests pay off. Spread a bit behind the ear or along the jaw before applying new combinations across the face. Dermatologists and serious skincare fans agree: less is often more, especially when trying out potent pigment correctors. Instead of rushing, introducing new actives one by one clears up whether the skin tolerates them well. That sort of patient approach often leads to healthier skin, fewer bad surprises, and brighter results.
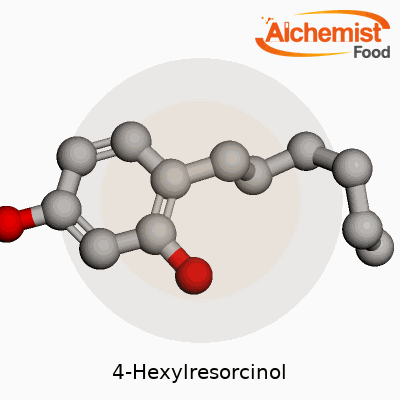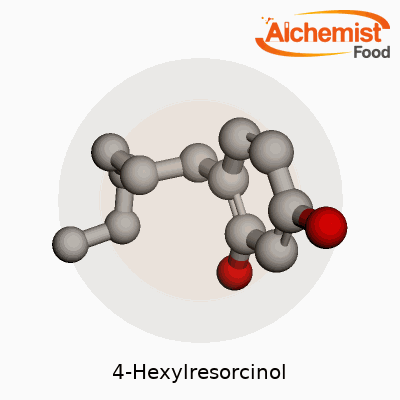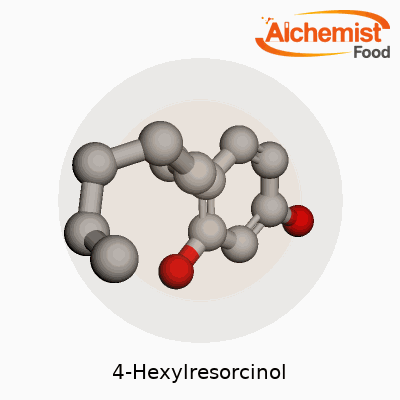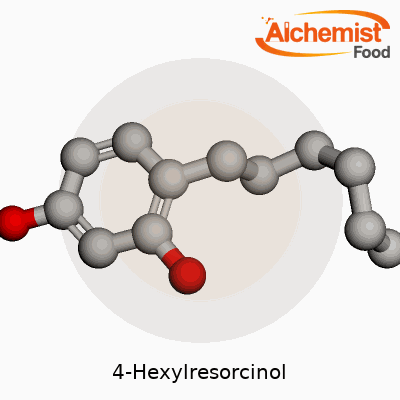
| Names | |
| Preferred IUPAC name | 4-Hexylbenzene-1,3-diol |
| Other names |
4-n-Hexylresorcinol Cremol mbvo Resorcinol, 4-hexyl- Hexylresorcinol Pfizerment Septisol n-Hexylresorcinol |
| Pronunciation | /ˌfɔːrˌhɛksɪl.rɪˈzɔːrsɪnɒl/ |
| Preferred IUPAC name | 4-hexylbenzene-1,3-diol |
| Other names |
1,3-Dihydroxy-4-hexylbenzene p-Hexylresorcinol Crestin Resorcinol, 4-hexyl- 4-n-Hexylresorcinol |
| Pronunciation | /ˌfɔːrˌhɛksɪl.rɪˈzɔːr.sɪn.ɒl/ |
| Identifiers | |
| CAS Number | 136-77-6 |
| Beilstein Reference | 1321340 |
| ChEBI | CHEBI:38464 |
| ChEMBL | CHEMBL1405 |
| ChemSpider | 13144 |
| DrugBank | DB11135 |
| ECHA InfoCard | ECHA InfoCard: 100.009.776 |
| EC Number | 204-714-2 |
| Gmelin Reference | 8796 |
| KEGG | C10635 |
| MeSH | D006634 |
| PubChem CID | 5377 |
| RTECS number | VL2450000 |
| UNII | 1R4A77J943 |
| UN number | UN3077 |
| CAS Number | 136-77-6 |
| Beilstein Reference | 1208730 |
| ChEBI | CHEBI:5720 |
| ChEMBL | CHEMBL1423 |
| ChemSpider | 7149 |
| DrugBank | DB09036 |
| ECHA InfoCard | 100.015.604 |
| EC Number | 1.14.18.1 |
| Gmelin Reference | 8826 |
| KEGG | C06505 |
| MeSH | D006520 |
| PubChem CID | 31246 |
| RTECS number | VL4375000 |
| UNII | 2Y481K52C7 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C12H18O2 |
| Molar mass | 194.28 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.1 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 3.65 |
| Vapor pressure | 8.2 × 10⁻⁷ mmHg (25°C) |
| Acidity (pKa) | 9.3 |
| Basicity (pKb) | 13.94 |
| Magnetic susceptibility (χ) | -80.0·10^-6 cm³/mol |
| Refractive index (nD) | 1.590 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.51 D |
| Chemical formula | C12H18O2 |
| Molar mass | 246.36 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.058 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 3.39 |
| Vapor pressure | 0.0000147 mmHg at 25°C |
| Acidity (pKa) | 10.10 |
| Basicity (pKb) | 13.97 |
| Magnetic susceptibility (χ) | -77.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.597 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.64 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -362.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3755.6 kJ/mol |
| Std molar entropy (S⦵298) | 393.06 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -370.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3937.8 kJ/mol |
| Pharmacology | |
| ATC code | D08AX04 |
| ATC code | D08AX04 |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| Flash point | 127°C |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | LD50 oral rat 730 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 740 mg/kg |
| NIOSH | RLQ8341DZN |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Hexylresorcinol: "Not established |
| REL (Recommended) | 0.1% |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: - |
| Flash point | 113°C |
| Autoignition temperature | 270°C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 2200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3700 mg/kg (rat, oral) |
| NIOSH | RN:136-77-6 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 5 mg/kg bw |
| Related compounds | |
| Related compounds |
Resorcinol 2-Methylresorcinol 4-Butylresorcinol 4-Ethylresorcinol 4-Propylresorcinol |

