

Mechanism
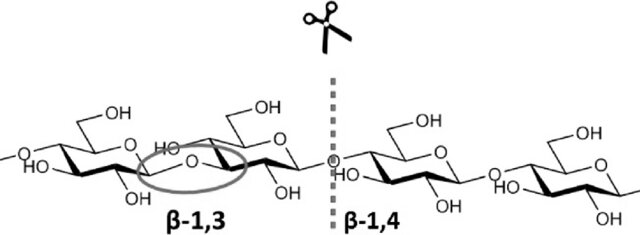
Figure 3Mode of action of endo-1,3-1,4-β-glucanase[1]
i.Mode of Action
The hydrolytic mechanism of β-glucanases, particularly those from Bacillus species such as Bacillus licheniformis, follows a double-displacement reaction. This involves two main steps: glycosylation and deglycosylation[2]. During glycosylation, a catalytic nucleophile attacks the anomeric carbon of the substrate, forming a covalent enzyme-substrate intermediate. This is followed by deglycosylation, where a water molecule, activated by a general acid-base, attacks the intermediate, completing the hydrolysis and releasing the product[2].
ii.Catalytic Residues
In the case of Bacillus 1,3-1,4-β-glucanases, which are retaining endo-glycosidases of family 16 GH, the catalytic mechanism involves a catalytic triad. This triad consists of two glutamic acid residues, Glu134 and Glu138, and an aspartic acid residue, Asp136[2]. Glu134 serves as the enzyme nucleophile, Glu138 acts as the general acid-base, and Asp136 assists in catalysis by participating in both glycosylation and deglycosylation steps, contributing to the pKa modulation of Glu138 during the enzyme cycle[2].
iii.Substrate Binding and Specificity
The binding-site cleft of Bacillus 1,3-1,4-β-glucanases is composed of several subsites, typically ranging from -4 to +2, with subsite -3 making the largest contribution to transition state stabilization[3]. The specificity of this subsite is crucial for both glycosidase and glycosynthase activities. For example, a D-galactosyl residue on the nonreducing end of a trisaccharide substrate can be accepted by the enzyme and binds at subsite -3 in the productive enzyme–substrate complex[3]. This specificity allows the enzyme to catalyze the hydrolysis of substrates with different glycosidic linkages, as well as to participate in transglycosylation reactions when the catalytic nucleophile is mutated[3].
iv.Transglycosylation and Glycosynthase Activity
Upon mutation of the catalytic nucleophile Glu134 to alanine, the Bacillus licheniformis 1,3-1,4-β-glucanase becomes a highly efficient endo-glycosynthase, with strict specificity for the formation of β-1,4 glycosidic bonds[2]. This modified enzyme can be used to catalyze the transfer of glycosyl residues to acceptor molecules, a process known as transglycosylation, which is valuable for oligosaccharide synthesis[2].
References
1. Edison, L.K., S. Shiburaj, and N. Pradeep, Microbial beta glucanase in agriculture, in Advances in Microbial Biotechnology. 2018, Apple Academic Press. p. 53-72.
2. Planas, A., M. Faijes, and M. Abel, Mechanism and Engineering of Bacterial 1,3-1,4-β-Glucanases : From Glucan Hydrolase to Glycosynthases in Enzymatic Oligosaccharide Synthesis. Journal of applied glycoscience, 2003. 50: p. 245-251.
3. Fairweather, J.K., et al., Specificity studies of bacillus 1,3-1,4-beta-glucanases and application to glycosynthase-catalyzed transglycosylation. Chembiochem, 2002. 3(9): p. 866-73.